CANNABIDIOL (CBD) AND THE ATHLETE: CLAIMS, EVIDENCE, PREVALENCE AND SAFETY CONCERNS
Published
March 2021
Author
Graeme L. Close, Ph.D.; Scott H. Gillham; Andreas M. Kasper
Topics
CBD , Recovery , Training & Performance , Supplements , Athlete Health

KEY POINTS
- Cannabidiol (CBD) is a non-psychotropic cannabinoid found in the cannabis plant.
- CBD is no longer prohibited by the World Anti-Doping Agency; however, all other cannabinoids remain on the prohibited list.
- The legal status of CBD is complicated and varies from country to country. The legal status in the United States is further complicated by differing state laws. Athletes and coaches must therefore be aware of the country (and state) specific legal status of CBD.
- Benefits of CBD have been reported both anecdotally and within the literature, with claims of anti-oxidative, anti-inflammatory, analgesic and neuroprotective properties, although studies on athletic populations are yet to be performed.
- Many athletes have previously or are currently supplementing with CBD products.
- The safety dataset of CBD is incomplete and known toxicities exist at pharmaceutical levels. However, anecdotal recreational use seems to suggest low risk of apparent side effects but this has not been tested in well-controlled studies. Additionally, there remains a significant risk of inadvertent doping via the presence of tetrahydrocannabinol (THC) or other cannabinoids in CBD supplements.
- Athletes should avoid the use of CBD and wait for further research into the efficacy and safety of supplementation.
INTRODUCTION
Nutraceutical and herbal remedies have been prevalent in many cultures for hundreds of years. The cannabis plant specifically has been utilized for the treatment of ailments and in a quest for pain relief. This plant contains > 140 cannabinoids with the most notable being ∆9-tetrahydrocannabinol (commonly referred to as THC) and cannabidiol (commonly referred to as CBD) (McCartney et al., 2020). THC has been identified as the main cannabinoid responsible for the psychotropic effect of cannabis and is therefore found at high concentrations in indica/marijuana (> 0.3%) and low concentrations in sativa/hemp (< 0.3%). CBD has been cited as a non-intoxicating constituent of the cannabis plant with potential therapeutic value (White, 2019).
In the past decade, concentrated CBD has started to become a more widely researched supplement for treatment of disorders, cited as providing anti-oxidative, anti-inflammatory analgesic and neuroprotective properties, in both sporting and clinical environments (reviewed in McCartney et al., 2020). Within the realm of elite sport, evidence suggests CBD use has increased exponentially (Docter et al., 2020; Kasper et al., 2020) which is likely due to both its removal from the World Anti-Doping Agency (WADA) prohibited list (WADA, 2018) alongside the evolving legal landscape.
The legal landscape is, however, complicated and varies from country to country and within the United States (U.S.) varies from state to state. In the United Kingdom (U.K.), CBD is currently legal to be sold as a supplement providing that the CBD comes from hemp, that the final product contains < 1 mg of THC, and that no medical claims are made surrounding its utility. The U.K. has also decided to classify CBD as a “novel food,” meaning companies wanting to continue to sell CBD will be required to have submitted and had their Novel Foods Application validated by 31st March 2021. Meanwhile, in the U.S., the 2018 Farm Bill was signed into law and removed hemp (defined as cannabis and derivatives of cannabis with no more than 0.3% THC on a dry weight basis) from the definition of marijuana in the Controlled Substances Act. While the Farm Bill opened potential commerce for hemp and its constituents, under the U.S. Food, Drug, and Cosmetics Act (FD&C Act), CBD and products containing CBD cannot be marketed or sold as dietary supplements or foods. This is because under the FD&C Act, it is unlawful to introduce into interstate commerce a product that contains or includes a substance that is an active ingredient in an approved drug product, (e.g., Epidiolex®, a prescription drug comprised of CBD isolate) or a substance for which substantial and public clinical investigations have been instituted. While the Food and Drug Administration has enforcement authority under the FD&C Act, it, to date, has only issued warning letters to companies making overt disease treatment or prevention claims. U.S. state laws may differ from federal laws, but federal laws may pre-empt and control, especially where interstate commerce is involved. Therefore, it is crucial for athletes and coaches to be fully aware of the country and state specific legislation before even considering the potential for CBD in sport.
Given the proposed therapeutic benefits associated with CBD, especially in relation to pain management, and the relatively recent legislative changes in sport, it is important that athletes and practitioners have an appreciation for the current claims, evidence, prevalence and safety concerns surrounding this herbal intervention. Therefore, the aims of this Sports Science Exchange article are to: 1) provide an overview of the endocannabinoid system (ECS); 2) provide an appraisal of the current evidence and efficacy in relation to CBD use in sport; and 3) highlight the issues and risks surrounding inadvertent doping following the use of CBD.
THE ROLE OF CBD WITHIN THE ENDOCANNIBINOID SYSTEM
The ECS plays an important role in the regulation of homeostasis within the body including being a vital modulator of the central and peripheral nervous systems, as well as the gastrointestinal tract, the endocrine, immune and reproductive systems (Figure 1). The ECS includes the endogenous cannabinoids (cannabinoids produced by the body, termed endocannabinoids), alongside cannabinoid receptors and enzymes that are required to synthesize and degrade these endocannabinoids. The two major endocannabinoids, anandamide and 2-arachidonoglycerol (2-AG), interact with cannabinoid receptors, namely CB1 and CB2 receptors, resulting in similar effects to those seen after taking the psychoactive constituents of the cannabis plant.
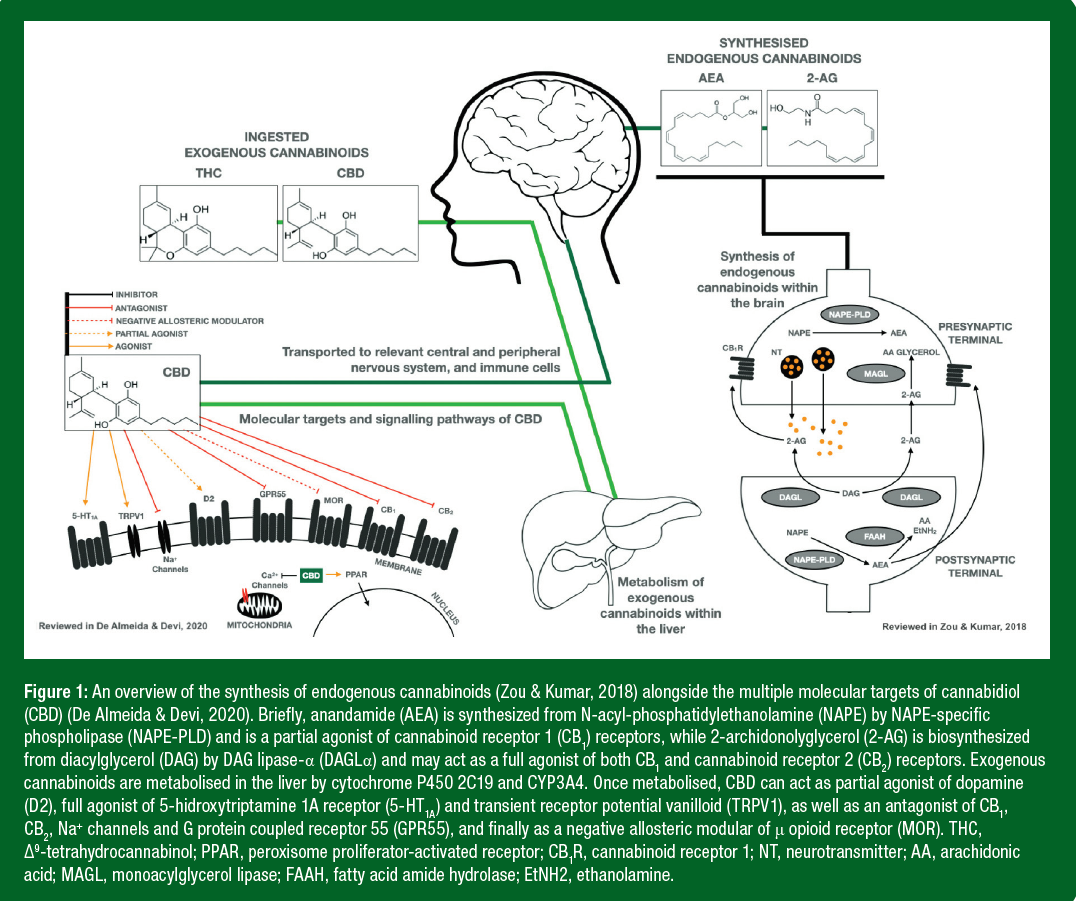
Once taken orally, cannabinoids are metabolized within the liver by cytochrome P450 2C19 and CYP3A4 (Zendulka et al., 2016). Like endocannabinoids, exogenous cannabinoids can directly trigger CB1 and CB2, activating various kinases and channels exerting a diverse range of physiological consequences (such as affecting synaptic function, gene transcription and cell motility) (Howlett et al., 2002). CB1 receptors consist of 472 amino acids in humans and are abundant within the peripheral and central nervous systems, cardiovascular system, gastrointestinal tract, skeletal muscle, liver and reproductive organs. Meanwhile CB2 receptors consist of 360 amino acids, and although present in many of the same systems as CB1 receptors, are mainly expressed within cells of the immune system (Zou & Kumar, 2018). Unlike THC, CBD does not bind to CB1 at the receptor site but binds to a separate allosteric site and acts as a negative modulator, making it more difficult for THC to bind to the CB1 receptor site (Tham et al., 2019). There is also emerging evidence that this may function as a partial agonist to CB2 (Tham et al., 2019). CBD has also been linked with many other non-endocannabinoid systems including interaction with serotonin, G protein-coupled, glycine, opioid and peroxisome proliferator activated receptors, along with various ion channels and enzymes (Ibeas Bih et al., 2015).
The precise molecular pathways and targets of CBD and the full mechanistic effects of THC, CBD and other less well-known cannabinoids in-vivo are yet to be fully established. Indeed, there is a suggestion of a potential synergistic “entourage effect” whereby other cannabinoids may cause additional effects (Russo, 2019), such as in patients with fibromyalgia suffering chronic pain (van de Donk et al., 2019). The perceived pain relief effect was shown in products containing CBD and THC as opposed to CBD alone and it is therefore important to ascertain the situational in-vivo benefits of CBD. The therapeutic potential of cannabinoids in a sporting context is particularly exciting given the ability to interact with the body’s own endocannabinoid system.
CLAIMS & EVIDENCE
Pain
Within the elite sporting environment where athletes are required to train and compete regularly, physical capacity and thus performance may be in part dependent on the athlete’s ability to complete multiple bouts of muscle damaging exercise. Consequently, symptoms of exercise induced muscle damage are common, including muscle soreness, aches, pains and inflammation (Owens et al., 2019). Nutrition is one avenue that has been shown to attenuate acute muscle damage, with common nutritional strategies including the provision of extra protein, amino acids, polyphenols, omega-3 fatty acids, creatine monohydrate and vitamins C, D and E (reviewed in Owens et al., 2019). These strategies often target reduced inflammation as a means of recovering muscle function; however, opiates and non-steroidal-anti-inflammatory drugs (NSAIDs) are also regularly taken within athletic environments for pain management (Tsitsimpikou et al., 2009). Whilst short term prescription of such pain-killing drugs has been reported to be safe (Morelli et al., 2017), when taken chronically, opiates such as tramadol and NSAIDs such as ibuprofen can cause nausea, headaches, constipation, sleep disturbances, gastric injury, gastric ulceration and kidney damage (Bertolini et al., 2001), as well as increasing the risk of bleeding and intracranial hemorrhage post minor head injury (Sakr & Wilson, 2005).
It is therefore not surprising that athletes are beginning to explore alternative pain-relieving therapies, one of which is CBD. Despite the mechanistic rationale, there is currently limited evidence to suggest CBD supplementation has anti-inflammatory effects in-vivo (Naftali et al., 2017), with much of the current research utilizing in-vitro pre-clinical models (Burstein, 2015). Moreover, the majority of studies investigating the effects of CBD on models of pain have been completed in animal models (Casey et al., 2017). That said, the findings of these studies show promise, with the suggestion that a co-administered dose of CBD and THC can reduce the allodynia associated with neuropathic pain in C57BL/6 mice (Casey et al., 2017). Given the current limitations of the literature and lack of evidence in human participants, it is too early to support the use of CBD as an alternate to standard pain medication in athletes. Studies investigating any potential beneficial effects of CBD on sport related muscle soreness are needed, including studies to establish an effective dose. More importantly, given the paucity of controlled safety data at subclinical doses, it is unknown if chronic CBD supplementation is safe, and whether drug-drug interactions exist, if sensitive subpopulations exist, and if the effective dose(s) overlap with those known to cause clinical toxicities at higher levels.
Sleep
Appropriate quality and quantity of sleep are vital for humans to facilitate normal daily function as well as recovery following exercise, with a lack of sleep resulting in impairments to psychological (cognition and well-being) and physiological processes (growth and repair of cells, metabolism of glucose and immune response) (Walsh et al., 2020). Indeed, disruption of sleep is seemingly common within athletic populations, specifically around competition, characterized by < 7 hours of sleep, sleep dissatisfaction, unrefreshing sleep, long sleep onset latency and daytime sleepiness and fatigue, with this being more prevalent in athletes than the general population (Walsh et al., 2020). Despite case study research suggesting that CBD supplementation (25-160 mg/day) may improve perceived sleep quality and disordered sleep disruptions (Chagas et al., 2014), a placebo-control, double blind cohort study has suggested no beneficial or even negative effects of CBD on sleep (Linares et al., 2018). Collectively, the evidence surrounding the efficacy of CBD supplementation on sleep is, at best, equivocal (McCartney et al., 2020). Therefore, its use is not suggested until there is a substantial body of sport-specific evidence to confirm its efficacy. Future studies should recruit physically active people, in order to develop a sound understanding of the effects of CBD supplementation on sleep quality.
Anxiety
Competition induced stress has been associated both directly and indirectly with poor athletic performance via over-arousal, decreased appetite (and thus insufficient energy intake) and loss of sleep (Craft et al., 2003). McCartney et al. (2020) reviewed the available research on the effect of CBD on subjective anxiety in healthy individuals and individuals with social anxiety and found that CBD had little influence on anxiety levels in low stress conditions. They did however find that studies investigating CBD supplementation (300-600 mg/day) during stress inducing conditions, both in healthy and socially anxious individuals, had equivocal results. These data suggest a potential for CBD to have anxiolytic effects during stressful situations although this needs to be further explored in athletic situations.
Concussion
Mild traumatic brain injury (such as concussion) may occur when training or competing in both contact and non-contact sports, usually caused by a blow to the head or violent shaking such as in a motor vehicle collision, collision based tackling and physical contact in combat sports. This injury results in detrimental changes in neurochemicals alongside distinct physical and neurological symptoms such as headaches, dizziness, nausea, difficulty balancing, poor motor coordination, disorientation, and anxious, aggressive and depressive behaviours. To date, only one study has directly investigated the effects of CBD supplementation on the treatment of traumatic brain injury, and this was in mice (1.5 mg/kg body mass, or ~3 μg/day, which roughly equates to ~0.85 mg/kg or 51 mg/day in humans). It was reported that CBD attenuated the changes in neurological behaviours alongside biochemical changes usually observed during post-brain insult potentially via decreased inflammation, oxidative stress and excitotoxicity (Belardo et al., 2019). The exact mechanisms by which CBD may act as a neuroprotective agent is not fully understood, and much work needs to be done in this field. Given the multiple risks to health associated with concussion in athletes, coupled with these potential neuroprotective effects at a reasonable human equivalent dose, it is vital that future research should investigate the effects of CBD supplementation in humans, particularly those at risk of brain injury through sport.
PREVALENCE OF CBD USE IN SPORT
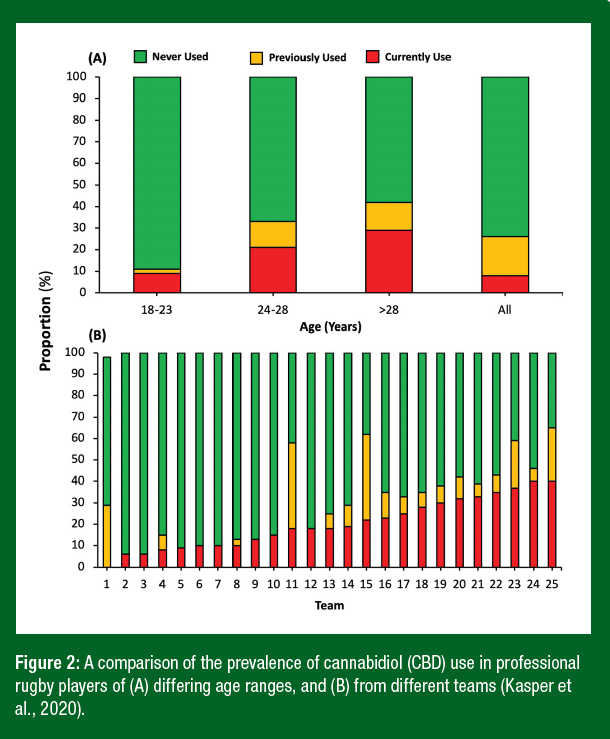
Despite widespread reports of CBD use by athletes in the mainstream media, and indeed several high-profile sport’s governing bodies, team and individual athletes now being sponsored by CBD manufacturers, published data of the magnitude of CBD use in professional sport are still lacking. We recently recruited over 500 professional rugby players in Europe to complete an anonymous survey on the prevalence of CBD use and the reasons why some athletes were turning to it (Kasper et al., 2020). Despite most teams in the sport of rugby advising against the use of CBD (due to inadvertent doping risks and lack of data on its efficacy), more than 25% of all players had either previously or currently used CBD (Kasper et al., 2020). Moreover, in the older players (> 28 yr old), almost 40% had or continue to use CBD with the major reasons cited including pain relief/recovery and to improve sleep quality (Figure 2A). Alarmingly, the players in this study reported that the major source of CBD information was either the internet (73%) or from a teammate (61%), with only 16% and 4% sourcing their information from the team nutritionist or other staff member, respectively (Kasper et al., 2020). This low reliance on the team nutritionist could be due to most clubs and governing bodies advising against the use of CBD and, as a consequence, players not feeling comfortable to discuss the potential use of CBD with internal staff members. This is perhaps the reason why some teams reported the prevalence of use as high as 60% which may reflect an influential individual within that team promoting its use (Figure 2B.). Taken together, these data suggest that many athletes may be willing to accept the risks associated with CBD in an attempt to accelerate recovery and/or improve sleep whilst simultaneously decreasing the use of standard analgesic medication which can be addictive and have serious side effects. A key take-home message from this research was that athlete support personnel must create environments whereby athletes feel comfortable to discuss CBD use with qualified staff rather than relying on the internet and/or other team members for their information.
SAFETY CONCERNS
While CBD use continues to increase, there remains a paucity of well controlled safety studies, particularly at doses relevant for recreational or self-medicating doses. The majority of safety data that exists in the public domain comes from preclinical and clinical studies completed investigating a pharmaceutical CBD product, Epidiolex. While the Epidiolex dataset highlights a number of safety concerns, it is important to acknowledge that the doses in a pharmaceutical context are typically much higher than those used recreationally or through self-medication. This alone does not mean these doses have been evaluated and deemed safe, but rather that there is still research needed to reconcile the safety concerns seen at high pharmaceutical doses relative to the lack of knowledge on safety at lower, recreational doses.
Anti-Doping Rule Violation risks
Despite CBD being removed from the WADA prohibited list in 2018, the use of CBD still poses a significant risk to athletes due to the possibility of THC and other prohibited cannabinoids being present in quantities sufficient to produce a urine sample greater than the current threshold. It is important to remember that THC is prohibited as a threshold compound meaning that any analytical finding > 150 ng/ml in the urine constitutes an anti-doping rule violation (ADRV) (Mareck et al., 2020; WADA, 2018). Many CBD products now state 0% THC on the label, but it should be stressed that all cannabinoids, except CBD, remain prohibited by WADA and therefore athletes must be sure that the CBD product is not just THC free, but also free from all other cannabinoids. It could be argued that cannabinoids other than THC pose a more serious risk given that these are not threshold compounds and technically any concentration in the urine could result in an ADRV. This is especially concerning for athletes using full-spectrum CBD products, which likely contain some THC along with other minor cannabinoids. What is of major concern is that it has recently been reported that only 15% of a selection of commercially available products in the U.S. were below the < 0.3% THC maximum limit (Gurley et al., 2020). Indeed, following a 4-week supplementation period of CBD (30 mg/day) with a reported concentration < 1 mg THC, urinary THC-COOH metabolite concentrations were detectable in 50% of participants (Dahlgren et al., 2020). This uncertainty of cannabinoid concentrations within commercial products is concerning for nutritionists and athletes alike and poses a serious risk in terms of an ADRV. In addition, given that rates of supplement contamination which could result in an ADRV have been reported as high as 12-58% (Martínez-Sanz et al., 2017), and batch testing of THC containing products is limited, there is a genuine risk of inadvertent doping by athletes. Finally, there are reports that exercise (Wong et al., 2013) or fasting (Gunasekaran, et al., 2009) can result in the release of stored THC within adipose tissue in cannabis users resulting in reintoxication. It is still unknown if very small doses of THC, perhaps those found in typical CBD supplements, can accumulate in athletes, and consequently increase to a concentration that would pose an ADRV risk following exercise or fasting. All of these questions need to be fully explored prior to recommending the use of CBD to athletes. One potential alternative to plant-based CBD which would reduce the risk of an ADRV from other cannabinoids is the use of synthetic CBD products which are becoming increasingly popular. Research however on these CBD products is even more scarce than plant-based CBD and given the potential entourage effect, studies will need to be performed on the efficacy, safety and optimal doses of synthetic CBD products prior to be considered by athletes. The solution to the anti-doping concerns may lie with the authorities responsible for anti-doping controls. Since WADA removed CBD from the prohibited list, yet left all other cannabinoids as prohibited substances, there has been serious potential for accidental ADRVs in athletes wishing to try CBD products. It has recently been announced that in the Ultimate Fighting Championship (UFC), the U.S. Anti-Doping Agency (USADA) will no longer be testing for cannabis as a prohibited substance providing the athlete does not look visibly intoxicated leading into the fight, whilst in the National Basketball Association (NBA) random testing for cannabis has been suspended for the 2020/21 season and rather focuses upon performance enhancing substances. It will be interesting to observe if this trend spreads to other sports.
PRACTICAL APPLICATIONS
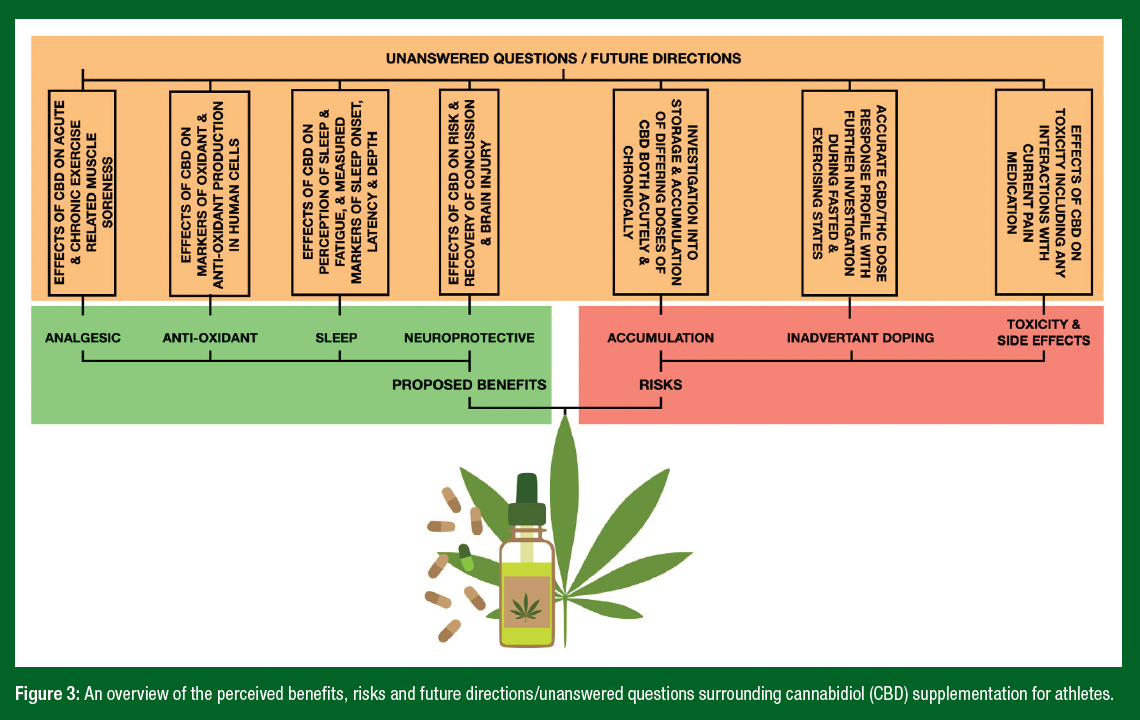
- The proposed benefits and unanswered questions with regards to CBD supplements and athletes are summarized in Figure 3.
- Practitioners should be aware that the emerging evidence suggests that many athletes are using CBD on a regular basis to help with pain management and improved sleep. It is therefore crucial that practitioners educate themselves on the science of CBD and fully understand the complexity of this product when it comes to the potential for an ADRV.
- The use of CBD appears to increase with age, suggesting older athletes are searching for enhanced pain management. Moreover, some teams appear to have key influencers resulting in a high prevalence of use. It is therefore crucial that these influencers are targeted for education.
- Despite many claims with regards to pain management, improved sleep, improved anxiety and concussion recovery, solid evidence is lacking, especially using doses that would be advised in non-clinical situations and in athletic populations.
- CBD appears to have a relatively safe adverse effects profile (in low doses; Chesney et al. 2020), however there are outstanding safety questions that should be addressed before chronic use can be deemed safe.
- Given that CBD products are likely to contain other cannabinoids which remain prohibited by WADA, the use of CBD at present remains a high risk for an ADRV.
SUMMARY
CBD has rapidly appeared as a suggested supplement for athletes promoted to help with sleep, recovery and anxiety. Given that this market has been predicted to be worth ~$10 billion by 2025 there is no reason to think that this supplement is not here to stay. Emerging research has suggested that despite many unanswered questions with regards to the efficacy of its use, safety profile, risk of an ADRV, legal status and even basic questions like, what is a suggested therapeutic dose, many athletes have become early adopters and now routinely use CBD as part of their recovery strategies. While this is of course a concern for practitioners, it is an exciting time for researchers given the significance of the endocannabinoid system and the potential that CBD has to interact with this important, yet understudied, physiological system. The current advice to athletes should be one of caution, or even abstinence, but we would strongly encourage researchers from many sport sciences disciplines to study this fascinating supplement to fully understand if the cannabis sativa L-strain does indeed hold the secret to helping athletes manage their day-to-day pain with minimal side effects (Figure 3).
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc. The authors would like to thank Dr. Douglas Kalman and Travis Schmit for their advice with regards to the legal status and safety of CBD.
REFERENCES
Belardo, C., M. Iannottta, S. Boccella, R. Cristina Rubino, F. Ricciardi, R. Infantino, G. Pieretti, L. Stella, S. Paino, I. Marabese, R. Maisto, L. Luongo, S. Maione, and F. Guida (2019). Oral cannabidiol prevents allodynia and neurological dysfunctions in a mouse model of mild traumatic brain injury. Front. Pharmacol. 10:352.
Bertolini, A., A. Ottani, and M. Sandrini (2001). Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol. Res. 44:437–450.
Burstein S. (2015). Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg. Med. Chem. 23:1377–1385.
Casey, S.L., N. Atwal, and C.W. Vaughan (2017). Cannabis constituent synergy in a mouse neuropathic pain model. Pain 158:2452–2460.
Chagas, M.H., A.L. Eckeli, A.W. Zuardi, M.A. Pena-Pereira, M.A. Sobreira-Neto, E.T. Sobreira, M.R. Camilo, M.M. Bergamaschi, C.H. Schenck, J.E. Hallak, V. Tumas, and J.A. Crippa (2014). Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J. Clin. Pharm. Therap. 39:564–566.
Chesney, E, D. Oliver, A. Green, S, Sovi, J. Wilson, A. Englund, A.P. Freeman, and P. McGuire (2020). Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 45:1799-1806.
Craft, L., M. Magyar, B. Becker, and D. Feltz, D (2003). The relationship between the Competitive State Anxiety Inventory-2 and sport performance: A meta-analysis. J. Sport Exerc. Psychol. 25:44-65.
Dahlgren, M.K., K.A. Sagar, A.M. Lambros, R.T. Smith, and S.A. Gruber (2020). Urinary tetrahydrocannabinol after 4 weeks of a full-spectrum, high-cannabidiol treatment in an open-label clinical trial. J. Am. Med. Assoc. Psych. Online ahead of print. PMID: 33146684.
de Almeida, D.L., L.A. Devi (2020). Diversity of molecular targets and signaling pathways for CBD. Pharmacol Res Perspect. 8:682-692.
Docter, S., M. Khan, C. Gohal, B. Ravi, M. Bhandari, R. Gandhi, and T. Leroux (2020). Cannabis use and sport: A systematic review. Sports Health 12:189–199.
Gunasekaran, N., L.E. Long, B.L. Dawson, G.H. Hansen, D.P. Richardson, K.M. Li, J.C. Arnold, and I.S. McGregor (2009). Reintoxification: The release of fat-stored ∆9-tetrahydrocannabinol (THC) into blood is enhanced by food deprivation or ACTH exposure. Br. J. Pharmacol. 158:1330-1337.
Gurley, B.J., T.P. Murphy, W. Gul, L.A. Walker, and M. ElSohly (2020). Content versus label claims in cannabidiol (CBD)-containing products obtained from commercial outlets in the state of Mississippi. J. Diet. Supp. 17:599–607.
Howlett, A.C., F. Barth, T.I. Bonner, G. Cabral, P. Casellas, W.A. Devane, C.C. Felder, M. Herkenham, K. Mackie, B.R. Martin, R. Mechoulam, and R.G. Pertwee (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54:161–202.
Ibeas Bih, C., T. Chen, A.V.W. Nunn, M. Bazelot, M. Dallas, and J.B. Whalley (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12:699-730.
Kasper, A.M., S.A. Sparks, M. Hooks, M. Skeer, B. Webb, H. Nia, J.P. Morton, and G.L. Close (2020). High prevalence of cannabidiol use within male professional rugby union and league players: A quest for pain relief and enhanced recovery. Int. J. Sport Nutr. Exerc. Metab. Online ahead of print. PMID: 32732454.
Linares, I., F.S. Guimaraes, A. Eckeli, A. Crippa, A.W. Zuardi, J. Souza, J.E. Hallak, and J. Crippa (2018). No acute effects of cannabidiol on the sleep-wake cycle of healthy subjects: A randomized, double-blind, placebo-controlled, crossover study. Front. Pharmacol. 9:315.
Mareck, U., G. Fusshöller, H. Geyer, M.A. Huestis, A.B. Scheiff, and M. Thevis (2020). Preliminary data on the potential for unintentional anti-doping rule violations by permitted CBD use. Drug Test. Anal. Online ahead of print. PMID: 33125823.
Martínez-Sanz, J.M., I. Sospedra, C.M. Ortiz, E. Baladía, A. Gil-Izquierdo, and R. Ortiz-Moncada (2017). Intended or unintended doping? A review of the presence of doping substances in dietary supplements used in sports. Nutrients 9:1093.
McCartney, D., M.J. Benson, B. Desbrow, B., C. Irwin, A. Suraev, and I.S. McGregor (2020). Cannabidiol and sports performance: A narrative review of relevant evidence and recommendations for future research. Sports Med. Open 6:27.
Morelli, K.M., L.B. Brown, and G.L. Warren (2017). Effect of NSAIDs on recovery from acute skeletal muscle injury: A systematic review and meta-analysis. Am. J. Sport Med. 46:224–233.
Naftali, T., R. Mechulam, A. Marii, G. Gabay, A. Stein, M. Bronshtain, L. Laish, F. Benjaminov, and F.M. Konikoff (2017). Low-dose cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Digest. Dis. Sci. 62:1615–1620.
Owens, D.J., C. Twist, J.N. Cobley, G. Howatson, and G.L. Close (2019). Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 19:71–85.
Rosenkrantz, H., Fleishman, R. W, and Grant, R. J. (1981). Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol. Appl. Pharma. 58: 118-131
Russo E.B. (2019). The case for the entourage effect and conventional breeding of clinical cannabis: no "strain," no gain. Front. Plant Sci. 9:1969.
Sakr, M., and L. Wilson (2005). Best evidence topic report. Aspirin and the risk of intracranial complications following head injury. Emerg. Med. J. 22:891–892.
Tham, M., O. Yilmaz, M. Alaverdashvili, M. Kelly, E.M. Denovan-Wright, and R.B. Laprairie (2019). Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 176:1455–1469
Tsitsimpikou, C., A. Jamurtas, K. Fitch, P. Papalexis, and K. Tsarouhas (2009). Medication use by athletes during the Athens 2004 Paralympic Games. Br. J. Sports Med. 43:1062–1066.
van de Donk, T., M. Niesters, M.A. Kowal, E. Olofsen, A. Dahan, and M. van Velzen (2019). An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 160:860–869.
Walsh, N.P., S.L. Halson, C. Sargent, G.D. Roach, M. Nédélec, L. Gupta, J. Leeder, H.H. Fullagar, A.J. Coutts, B. J. Edwards, S.A. Pullinger, C.M. Robertson, J.G. Burniston, M. Lastella, Y. Le Meur, C. Hausswirth, A.M. Bender, M.A. Grandner, and C.H. Samuels (2020). Sleep and the athlete: narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. Online ahead of print. PMID: 33144349.
White C.M. (2019). A review of human studies assessing cannabidiol's (CBD) therapeutic actions and potential. J. Clin. Pharmacol. 59:923–934.
Wong, A., M.E. Montebello, M.M. Borberg, K. Rooney, N. Lintzeris, R. Bruno, J. Booth, J.C. Arnold, and I.S. McGregor (2013). Exercise increases plasma THC concentrations in regular cannabis users. Drug Alcohol Depend. 133:763-767.
World Anti-Doping Agency. Summary of Major Modifications and Explanatory Notes. 2018. https://www.wadaama.org/sites/default/files/prohibited_list_2018_summary_of_modificactions_en.pdf.Accessed 20 Nov 2020.
Zendulka, O., G. Dovrtelová, K. Nosková, M. Turjap, A. Sulcová, L. Hanus, and J. Jurica (2016). Cannabinoids and cytochrome P450 interactions. Current Drug Metab. 17:206-226.
Zou, S., and U. Kumar (2018). Cannabinoid receptors and the endocannabinoid system: Signalling and function in the central nervous system. Int. J. Mol. Sci. 19:833-856.








