Functional Ingredients to Support Active Women
Published
August 2022
Author
Abbie E. Smith-Ryan, PhD, CSCS*D, Hannah E. Cabre, MS, RD, Sam R. Moore, MS, CSCS
Topics
Sports Nutrition , Athlete Health , Female Athlete , Supplements

KEY POINTS
- Important sex-based differences exist between men and women that may influence nutrition and dietary supplement recommendations.
- Hormonal fluctuations throughout the menstrual cycle, and with oral contraceptives, result in metabolic alterations which should be considered when making supplement considerations for active women.
- Body composition and protein metabolism change throughout a woman’s lifespan and may be supported by sex-specific nutritional recommendations.
INTRODUCTION
Women are the largest consumers of dietary supplements with 77% of women utilizing at least one ingredient or supplement (CRN, 2020). Despite the large use of dietary supplements among women, the representation of women in research validating the use of these supplements is severely lacking (Cowley et al., 2021). Foundational research has highlighted physiological differences among males and females which may impact exercise and nutritional considerations for active women. Although sex-specific sport nutrition research falls far short of what is necessary, foundational female physiology combined with available dietary supplement evidence allows for a discussion of potential ingredients that may support active women and their goals. This Sports Science Exchange (SSE) article aims to provide an overview of physiological considerations for active women, that in turn may influence dietary supplement choices to support the needs of active women across the lifespan.
Important sex-based differences exist in metabolism (Tarnopolsky, 2008), fatigability (Hunter, 2014), vasodilation (Parker et al., 2008), and body composition (Bredella, 2017). Many, but not all, of these differences are driven by the varied hormonal landscape across a menstrual cycle and a woman’s lifespan. Estrogen is regarded as a master regulator of bioenergetic systems in the female body, and estrogen levels remain consistent from puberty into adulthood. The fluctuations of endogenous hormones across the menstrual cycle facilitate varying modulations in energy expenditure and macronutrient metabolism for regularly menstruating women (Moore et al., 2022). In the years preceding menopause, known as peri-menopause (average age 45 years), estrogen levels begin to dramatically decrease and menstrual cycles become irregular until the final menstrual period, known as menopause (average age 51 years) (Greendale et al., 1999). The decrease and eventual loss of estrogen has important implications for body composition and metabolism as active women age (Gould et al., 2022; Greendale et al., 2019).
As women transition from perimenopause to post-menopause, the loss of estrogen may decrease energy expenditure. In a one-year longitudinal study, 24-hour energy expenditure decreased significantly with age, and fat oxidation decreased by 32% in women who became postmenopausal compared to premenopausal (Lovejoy et al., 2008). There is also consistent evidence from basic and preclinical research that the disruption of estrogen signaling accelerates fat accumulation, and the loss of estrogen at menopause is likely to have pronounced effects on body composition (Van Pelt et al., 2015). These unfavorable alterations in body composition, which abruptly worsen at the onset of the menopause transition, indicate perimenopause may be a key period for exercise and nutrition interventions (Gould et al., 2022). This is especially so regarding maintenance of lean mass (LM), mitigating fat mass (FM) gain, and alterations in energy expenditure.
MENSTRUAL CYCLE
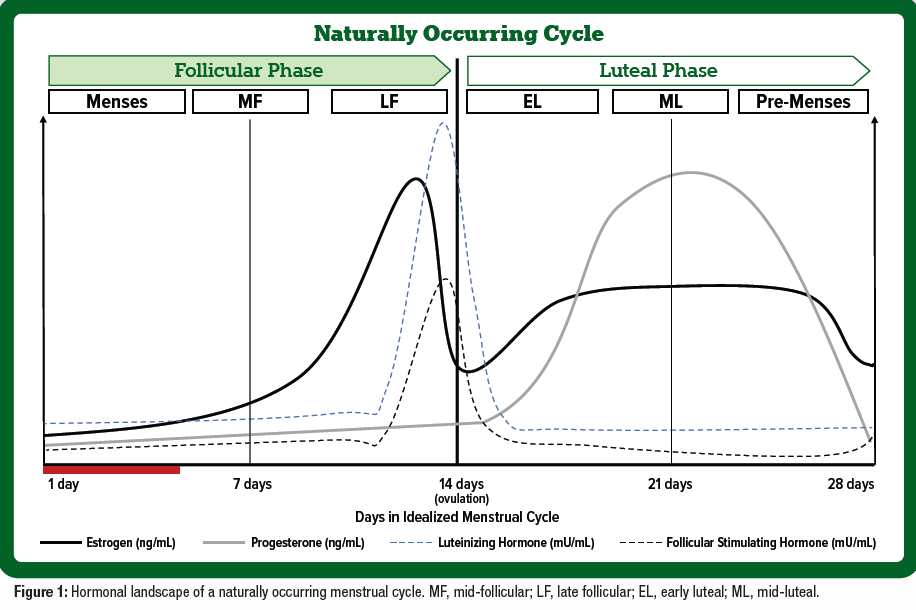
Between the ages of ~12 and 51 years old, females experience a circa mensal rhythm termed the menstrual cycle, characterized by predictable fluctuations in ovarian hormones, estrogen, and progesterone. A regular menstrual cycle can range from 23 – 38 days (Figure 1) and consists of three main phases: the follicular phase (FP), ovulation, and the luteal phase (LP) (Draper et al., 2018). The FP is characterized by low estrogen and progesterone levels as the FP ends, and with ovulation estrogen rises and falls with a spike in follicular stimulating hormone and luteinizing hormones. The latter half or LP of the cycle is characterized by a sharp rise in progesterone and higher levels of estrogen to prepare the body for pregnancy and ends with menstruation if pregnancy does not occur. These hormonal fluctuations are important considerations for eumenorrheic women who exercise as they may influence substrate utilization, performance, and recovery across the cycle.
ORAL CONTRACEPTIVES
To date, the majority of available research on women has evaluated naturally occurring menstrual cycles, excluding women who utilize hormonal contraception methods. However, more than 60% of adult women in the United States, and 57% of American collegiate female athletes, utilize some form of hormonal contraception (Kavanaugh & Jerman, 2018). Combination oral contraceptive (OC) pills, monophasic and triphasic, are the most common type of hormonal contraception prescribed, and the second most common hormonal profile in females. Monophasic OC is the prevailing dosage type, characterized by consistent amounts of ethinyl estradiol and progestin throughout the month. Triphasic OCs are distinguished by a consistent dosage of ethinyl estradiol while the synthetic progestin is delivered in low, moderate, and high amounts over three weeks, attempting to mimic the gradual onset of progesterone seen in a eumenorrheic menstrual cycle. Despite the prevalence of OC use in the athletic and general populations, the effects of OCs on exercise performance and metabolism are poorly understood. Furthermore, the differences in endocrine profiles among females (i.e., hormonal contraceptive users and non-users) highlight the need for future hormonal profile considerations within sport and exercise science research. Understanding the implications of OCs may clarify the opportunity for use of dietary supplements among women, as modulations in metabolism can be impacted by hormone dosages as well as progestin type. Based on existing physiological data, dietary supplements may help to modulate the inflammatory response, reduce muscle damage, and possibly lower oxidative stress among those utilizing OCs (Cauci et al., 2016). With limited data in this space, weighing the benefit-to-risk ratio of dietary supplements for these outcomes among women using OCs will be important.
BODY COMPOSITION
Sex-dependent differences in body composition begin at puberty primarily due to estrogen’s role in the regulation of body composition in females. Estrogen receptors are widely distributed across tissues such as adipose tissue and skeletal muscles (Gruber et al., 2002). Changes in energy expenditure across the female lifespan hold important implications for body composition. Estrogen receptors are located on the mitochondria, suggesting estrogen signaling may mediate the regulation of body composition and energy balance (i.e., energy expenditure and intake). For the active woman, stimulating muscle protein synthesis (MPS) is a key factor for the adaptive responses to exercise with implications for improvements in body composition, particularly LM. Some studies suggest there are small changes in protein kinetics during the LP, with greater utilization of protein at rest and with exercise (Draper et al., 2018; Kriengsinyos et al., 2004). In the transition from pre- to peri-menopause, preliminary data indicates a pronounced decrease in skeletal muscle protein balance (Gould et al., unpublished observations). Menopausal-related hormonal changes and losses in muscle mass may be a direct result of skeletal muscle dysregulation, due to the development of anabolic resistance to nutrient intake, particularly to the precursor essential amino acids (EAA) (Gould et al., unpublished observations; Katsanos et al., 2005).
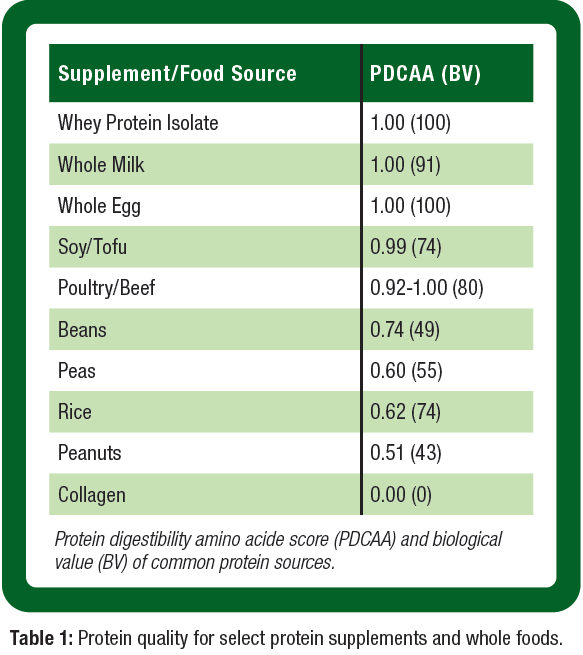
Due to the additional increased protein turnover associated with exercise, current sports nutrition guidelines for daily protein intake are 1.2 – 2.0 g/kg/day to optimize exercise training adaptations (Phillips et al., 2007). In female athletes specifically, very limited research has been conducted on the protein requirements, but existing data suggests that active women should consume a minimum of 1.6 g/kg/day of protein (Houltham & Rowlands, 2014). As a result of the potential blunted MPS response as women age, protein sources should consist primarily of higher bioavailable choices to maximize MPS (e.g., high digestibility, absorption, and essential amino acid content) (Table 1). This blunted sensitivity appears to be overcome when larger amounts of the EEAs, including leucine, are ingested suggesting an EAA supplement may be an important intervention to mitigate LM loss in active and aging women (Katsanos et al., 2005). Protein supplementation through a high-quality protein such as whey protein sources or EAA, can complement high-quality proteins consumed through the diet. When considering the distribution of macronutrients, the carbohydrate to protein ratio has been shown to be important for supporting optimal body composition and fat loss in women. The consumption of a ratio of 2:1 carbohydrate to protein has demonstrated significant losses in percent body fat while supporting a gain in LM (Layman et al., 2003; Lockwood et al., 2008).
DIETARY SUPPLEMENTS FOR ACTIVE FEMALES
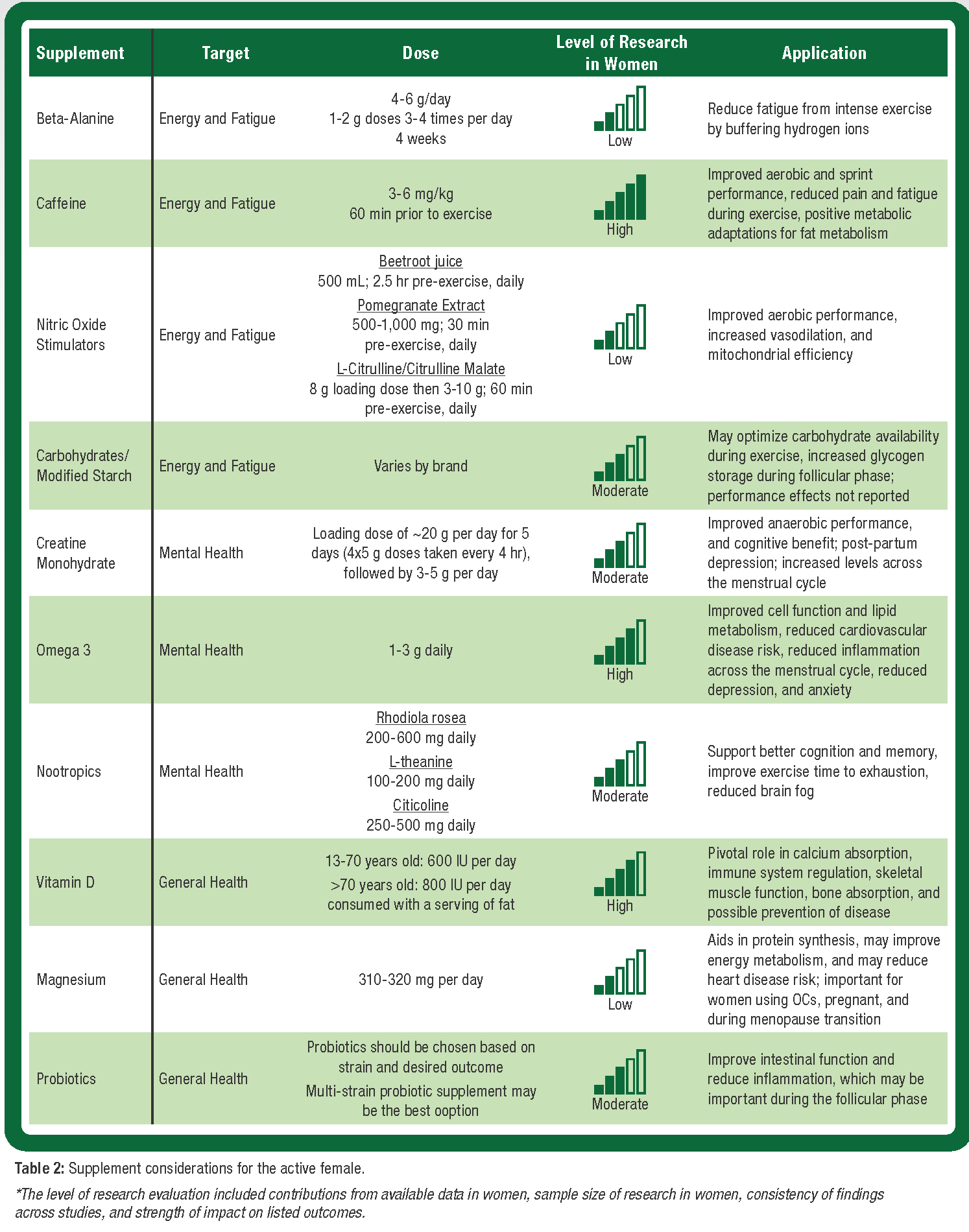
The most common reason women cite for use of dietary supplements is increased energy (CRN, 2020). Considering the lifestyle of active women, which often includes reported levels of multi-tasking, invisible labor, child-rearing, and a career, optimizing formulation and use of dietary supplements is warranted. When evaluating a dietary supplement, third-party testing is imperative to verify product contents and rule out contaminants. The most common and recommended certification programs to look for when identifying evidence-based supplements include; National Sanitation Foundation (NSF), NSF for Sport, Informed Choice, Consumer Labs, and the Banned Substances Control Group (BSCG). The United States Pharmacopeia (USP) also provides third-party verification for vitamins. Based on available evidence in women as well as a physiological foundation, there are dietary supplements and ingredients that may be beneficial (Wohlgemuth et al., 2021). Ingredients that target energy/fatigue, cognition/focus, and general health/deficiencies should be considered (Table 2). The authors also highly encourage future female-specific research in this space, and for practitioners, researchers, and active women to apply the information we have in hand, while continuing to experiment and innovate to better understand and meet the needs of active women.
Ingredients that may support an improvement in energy or delay fatigue for women
Beta-Alanine. Beta-alanine is a non-essential amino acid that has been shown to delay fatigue, particularly in activities lasting 2 - 4 minutes (Trexler et al., 2015) (Table 2). Beta-alanine is the rate liming substrate for carnosine synthesis, which acts as an innate hydrogen ion buffer in muscle during high-intensity exercise (Trexler et al., 2015). Consumption of beta-alanine is supported to be the most effective way to augment muscle carnosine concentration, which accounts for 15% of muscle buffering during intense exercise (Harris et al., 2006). Active women who are beginning a new exercise routine or having a hard time recovering from intense exercise may benefit from beta-alanine supplementation. Accurate dosing is imperative with beta-alanine, and to date, dosing does not appear to differ between men and women, although women are likely to have lower baseline muscle carnosine levels compared to men (Everaert et al., 2011). Increasing muscle carnosine helps to buffer hydrogen ions and maintain pH during exercise, thereby delaying fatigue, as well as increasing exercise intensity (Trexler et al., 2015). There is additional data that suggests beta-alanine may have some antioxidant properties (Smith-Ryan et al., 2014), which may be relevant for women during the FP of the menstrual cycle when oxidative stress may be elevated, to support greater recovery. With a 14-week half-life, beta-alanine is not something that needs to be taken all the time, but instead cycled on and off as needed.
Caffeine. Caffeine is one of the most widely studied and used ingredients, acting as a central nervous system stimulant (Table 2). There are physiological considerations for female-specific caffeine recommendations. Caffeine elimination appears to fluctuate over the course of the menstrual cycle with slower elimination and more pronounced effects during the LP as well as with OC use (Lane et al., 1992). The accumulation of caffeine during this high estrogen phase may magnify premenstrual symptoms, as well as intensify the sympathetic effects of caffeine, resulting in increased heart rate, anxiety, and impaired sleep. Caffeine is effective for improving repeated sprint/intermittent exercise performance and endurance performance, due to its ability to reduce pain perception and may augment fat oxidation during aerobic exercise, thereby sparing muscle glycogen (Guest et al., 2021). A typical recommended dose of 3 – 6 mg/kg consumed 60 min prior to exercise can delay fatigue/increase energy. Additionally, if a woman consumes caffeine habitually, a higher dose (6 mg/kg) or removing caffeine for a period of time may be needed to see significant improvements in anaerobic performance (Filip-Stachnik et al., 2021).
Creatine. The benefits of creatine supplementation for women are growing in evidence (Table 2). Changes in endogenous hormone concentrations may underpin varying creatine characteristics between males and females (Smith-Ryan et al., 2021), with females demonstrating 70 – 80% lower rates of creatine synthesis and consumption of considerably lower amounts of dietary creatine compared to men (Brosnan & Brosnan, 2007). Fluctuations in creatine kinase levels have been reported to be influenced by endogenous hormones, with the lowest concentrations observed during non-menstruating years, and subsequent decreases in creatine kinase activity with age and pregnancy. Creatine supplementation may be particularly effective post-partum as a result of cellular energy depletion surrounding childbirth (De Guingand et al., 2019). Short- and long-term creatine supplementation has shown significant beneficial ergogenic outcomes in strength, hypertrophy, and exercise performance in trained and untrained female populations when compared to placebo controls (Smith-Ryan et al., 2021). Mechanisms supporting increases in strength, hypertrophy, and performance, are likely related to increased intramuscular phosphocreatine stores which allow for greater stimulus of training through greater energy availability from increased adenosine triphosphate (ATP) turnover during exercise. Data also suggest positive relationships between mood and severity of depressive episodes with creatine and phosphocreatine levels in the brain (Dager et al., 2004). Creatine supplementation has also been shown to effectively reduce mental fatigue and improve cognitive performance, specifically during times of high stress or impacted sleep quantity or quality (Volz et al., 1998). A common misconception surrounding creatine supplementation pertains to undesirable weight gain in women, however, research shows initial gains incurred with loading doses are likely a result of increased cellular hydration (i.e., “water weight”), which can show favorable outcomes for hydration (Sobolewski et al., 2011). This change in fluid does not increase weight in women but instead increases extracellular fluid. Currently, supplement dosing appears to be the same for males and females (Smith-Ryan et al., 2021)
Teacrine. Teacrine is a newer compound, found in tea and coffee, that acts in a similar method as caffeine as an adenosine receptor antagonist. In addition to increased feelings of energy and a delay in fatigue, improvements in mood and cognition have also been reported with teacrine supplementation in a mixed sample of males and females (Bello et al., 2019). Teacrine has resulted in these effects without increases in heart rate or habituation. The combined effects of caffeine and teacrine may be a unique combinatory approach for increasing energy and delaying fatigue in women, yet more female-specific research with teacrine is warranted as current research has been mostly conducted in males (Cesareo et al., 2019; Kuhman et al., 2015).
Nitric Oxide Stimulators. Sex-based differences in physiology and biological processes may influence nitric oxide (NO) production (Wickham & Spriet, 2019) (Table 2). Nitrate products are thought to increase NO production through the nitric oxide synthase (NOS)-dependent pathway of NO production, which includes a series of reactions oxidizing L-arginine to L-citrulline and NO. Specifically, NO is a potent signaling molecule that elicits changes in biological and physiological processes such as vasodilation, mitochondrial efficiency, and calcium handling, all of which have important implications for exercise capacity (Jones et al., 2012). Compared to males, females have demonstrated increased blood flow during intermittent exercise. However, females have smaller vessels indicating they may be more likely to benefit from nitrate intake, particularly as it relates to vasodilation. Females also have a greater ability to reduce nitrates to NO compared to males, suggesting nitrate supplementation may be more effective in women compared to men (Wickham & Spriet, 2019). Supplementation with nitrates may be particularly important for aerobic activities and for delaying fatigue during exercise. More recently, acute and chronic supplementation with beetroot juice (280 ml/day) in young female OC users, did not improve aerobic performance but did improve torque production (Wickham et al., 2019). Additionally, 140 mL of beetroot juice consumed 2.5 h prior to exercise in trained females resulted in no effect on oxygen consumption but did reduce perceived exertion (Forbes & Spriet, 2022). The impact of dietary nitrate supplementation on recovery or across the menstrual cycle has not yet been explored in women.
Carbohydrates and Modified Starch. The importance of carbohydrate availability for exercise performance is well established (Jeukendrup, 2004) (Table 2). As a result of sex-based differences that exist in carbohydrate and fat oxidation during exercise (Boisseau & Isacco, 2021), as well as differing sensitivities of the gastrointestinal (GI) tract among active women (Godoy Reys & Gimenez-Sanchez, 2019), carbohydrate supplementation during exercise is ergogenic (Jeukendrup, 2004). In concert with carbohydrate feeding during exercise, symptoms of GI distress have been reported to be more prevalent in female endurance athletes, and among those women consuming hypotonic beverages (ten Haaf et al., 2014). Other forms of carbohydrate supplementation may be important to consider for active women, particularly for women undergoing endurance exercise. Modified starches may affect the gastric-emptying rate enhancing glycogen storage (Ormsbee et al., 2014) which may be beneficial during the FP, or spare glycogen by enhancing fat oxidation, which may also be beneficial as estrogen and progesterone levels change. To date, research has failed to demonstrate a positive effect of a fast-digesting high molecular weight starch in female cyclists (Mock et al., 2021) or a slow digesting modified starch (Ormsbee et al., 2014). There are some potential positive data with modified starch on performance, but only in men. This area needs additional study but addressing and modifying the carbohydrate source may be helpful for the active woman undergoing exercise activities that rely on muscle glycogen, as well as to mitigate GI distress.
Ingredients that may target cognition and focus
Omega 3. The essential fatty acids, omega 6 and omega 3, are key modulators of cell function, lipid-soluble vitamin absorption, and lipid metabolism (Table 2). The two most active eicosanoids derived from omega 3 are EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid), which play a vital role in improving immune function, reducing inflammation, and aiding in growth and development (Calder, 2006; Simopoulos, 2002). The reduction of systemic inflammation may be particularly beneficial during the FP of the menstrual cycle when systemic inflammation is elevated. Essential fatty acids have also demonstrated beneficial effects on mood (Freeman et al., 2006) and cognitive function (Robinson et al., 2010). Additionally, increased levels of omega 3 have been shown to reduce symptoms of depression and anxiety (Zhang et al., 2020), which are reported in higher rates in women vs. men.
Nootropics. There is a growing category of dietary supplements, ‘nootropics,’ that target cognition and memory, with some also supporting enhanced exercise outcomes (Table 2). Nootropics have gained even more traction among active women during the COVID-19 pandemic. The data reports greater levels of stress, and mental and sleep impairments among women, with other reports describing a cognitive-like ‘fog’ among women (Garrigues et al., 2020). Nootropics may support better cognition and memory, particularly among women. There are a few that have been substantiated, including rhodiola, L-theanine, ashwagandha (Bonilla et al., 2021; Choudhary et al., 2017), cordyceps (Yuan et al., 2018), choline/citicoline (McGlade et al., 2012), and bacopa monnieri (Stough et al., 2001). While this is not meant to be an exhaustive literature review, the above ingredients may support cognition in the active woman.
Ingredients for General Health & Wellness
Vitamin D. Vitamin D is traditionally known for its pivotal role in calcium absorption (Table 2). However, it also is imperative for innate and acquired immune system regulation, skeletal muscle function, bone absorption, and possible prevention of disease (Bohon & Goolsby, 2013). For active women, vitamin D levels could directly affect muscle strength and performance, recovery from exercise, and bone health. Furthermore, vitamin D deficiency has been shown to increase the risk of anemia, which is highly prevalent among active women (Sim et al., 2010). Vitamin D supplementation is recommended for women across the lifespan and across activity levels. Additionally, a focus on dietary vitamin D through foods such as fish, cheese, and some fortified cereals is an important consideration for women. Vitamin D is fat-soluble, which means it needs to be consumed with at least one serving of fat (IOM, 2005).
Magnesium. Magnesium activates enzymes involved in protein synthesis and several metabolic reactions and may improve energy metabolism (Volpe, 2013) (Table 2). Acute changes in magnesium concentrations are noticeable during a continuous bout of moderate to high-intensity exercise (Kerksick et al., 2018). There is growing evidence to support the essential role of magnesium in various physiological outcomes for women as they age (Porri et al., 2021). Specifically, there are various pathophysiological conditions across the female lifespan, such as the use of OCs, pregnancy, and menopause, which may increase magnesium requirements (Porri et al., 2021). Magnesium supplementation in pre-menopausal women may improve premenstrual syndrome (PMS) symptoms by decreasing inflammatory markers (Yonkers et al., 2008). In peri- to post-menopausal women, magnesium supplementation may be protective of bone health through optimization of vitamin D status (Kisters et al., 2020). Magnesium-rich foods include nuts, almonds, bananas, black beans, brown rice, cashews, spinach, seeds, and whole grains.
Probiotics. The GI tract of a woman begins to differ from a man at the onset of puberty and continues to change with hormonal fluctuations (Kim et al., 2020) (Table 2). Early evidence suggests that women have lower intestinal permeability and higher microbial diversity but are more sensitive to perturbation (Edogawa et al., 2018). Women also have reported greater symptoms of irritable bowel syndrome and leaky gut, particularly with exercise (Yang et al., 2021). Probiotics have been shown to be an effective stimulus for promoting bacterial diversity and targeting many aspects of health. Probiotic supplementation has also been shown to improve intestinal function and reduce inflammation, which may be effective for modulating changes in inflammation during the FP (Cristofori et al., 2021).
Other Vitamins & Minerals
Iron and Calcium. Iron deficiencies among active women are common, particularly among those that are menstruating. Active women should consume 18 mg/day of iron, with special attention being paid to vegan/vegetarian women, due to lower bioavailability of plant-based iron sources (Hunt, 2003). Female athletes have also reported lower calcium intake and calcium is essential for muscle contraction and bone development. The daily recommended intake of calcium for adult women is 1000 mg/day (Bendich, 2001).
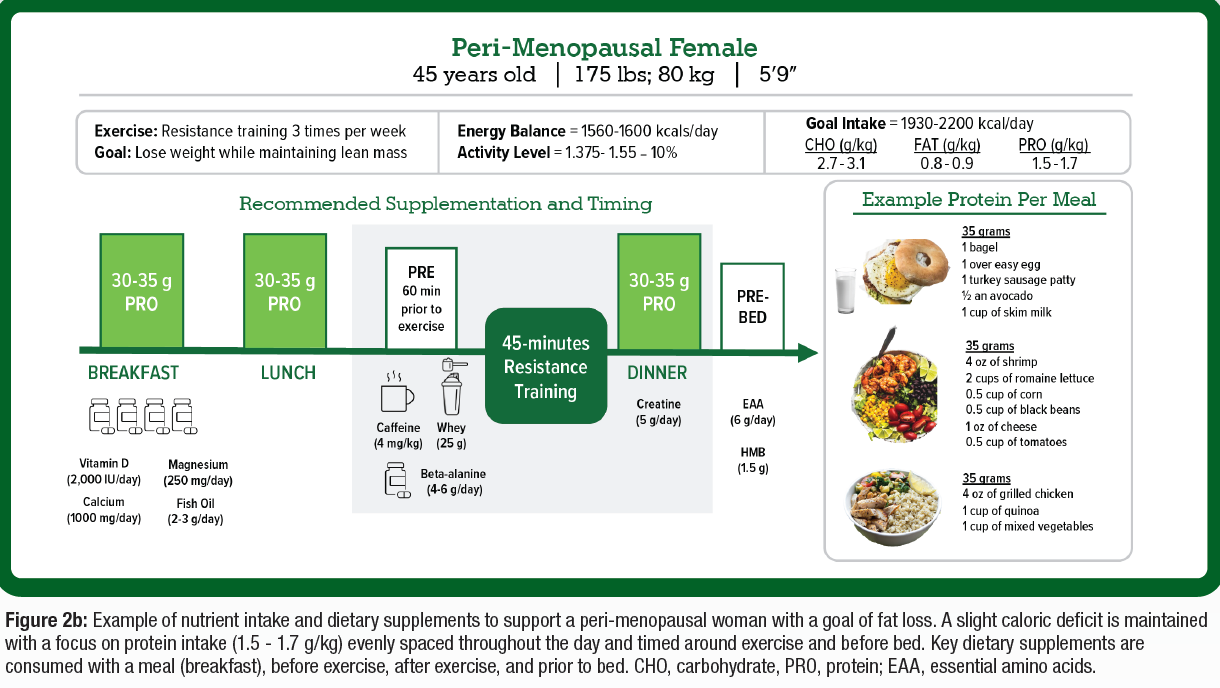
PRACTICAL APPLICATIONS
- Menstrual and menopause-related hormonal changes are important considerations for women who exercise as they may influence primary energy sources, performance, and recovery.
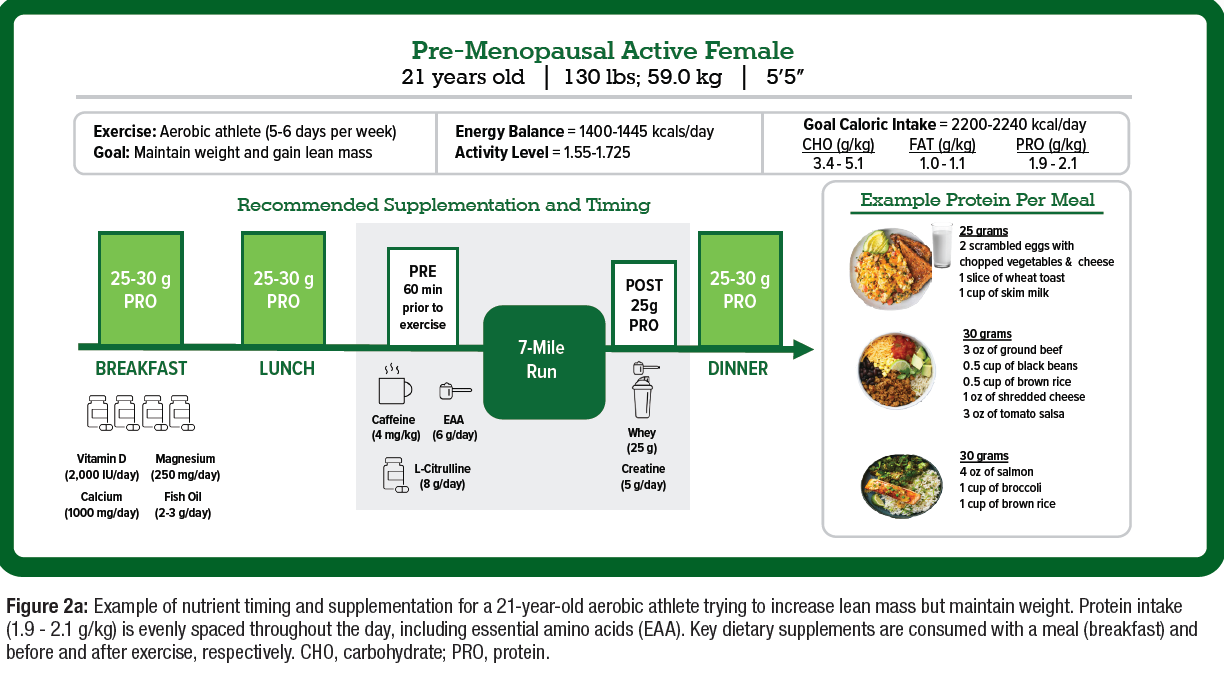
- Nutritional support and modifications can help to optimize lean body mass and improve body composition for active women, which may differ between sexes (Figure 2A).
- Available evidence combined with physiological rationale supports specific ingredients to optimize body composition, delay fatigue, and improve mental and physical health among active women (Figure 2A & 2B).
- While there is a need for more female-specific dietary supplement research, based on the available science, there are ingredients that may provide advantages for active women with the desire of delaying fatigue and increasing energy around exercise and in day-to-day life (Figure 2A & 2B).
SUMMARY
Women have unique nutritional and physiological needs. Based on available data and physiological understanding of the menstrual cycle and OC use, education for dietary supplements among women is essential. While there is a need for more research to evaluate the use of ingredients on the health and performance of women, there is enough evidence when combined with a physiological foundation to support specific ingredients to optimize body composition, delay fatigue, and improve mental and physical health among women. Future research and product development must include women across the lifespan and begin to expand upon their needs to improve health, quality of life, and performance.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Bello, M.L., A.J. Walker, B.A. McFadden, D.J. Sanders, and S.M. Arent (2019). The effects of TeaCrine(R) and caffeine on endurance and cognitive performance during a simulated match in high-level soccer players. J. Int. Soc. Sports Nutr. 16:20.
Bendich, A. (2001). Calcium supplementation and iron status of females. Nutrition 17:46-51.
Bohon, T.M., and M.A. Goolsby (2013). The role of vitamin d supplements in women's health. Clin. Med. Insights Womens Health, 6:67-70.
Boisseau, N., and L. Isacco (2021). Substrate metabolism during exercise: sexual dimorphism and women's specificities. Eur. J. Sport Sci. 22:672-683.
Bonilla, D.A., Y. Moreno, C. Gho, J.L. Petro, A. Odriozola-Martinez, and R.B. Kreider (2021). Effects of Ashwagandha (Withania somnifera) on physical performance: systematic review and bayesian meta-analysis. J. Funct. Morphol. Kinesiol. 6:20.
Bredella, M.A. (2017). Sex differences in body composition. Adv. Exp. Med. Biol. 1043:9-27.
Brosnan, J.T., and M.E. Brosnan (2007). Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 27:241-261.
Calder, P.C. (2006). n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83(6 Suppl):1505S-1519S.
Cauci, S., C. Buligan, M. Marangone, and M.P. Francescato (2016). Oxidative stress in female athletes using combined oral contraceptives. Sports Med. Open 2:40.
Cesareo, K.R., J.R. Mason, P.G. Saracino, M.C. Morrissey, and M.J. Ormsbee (2019). The effects of a caffeine-like supplement, TeaCrine(R), on muscular strength, endurance and power performance in resistance-trained men. J. Int. Soc. Sports Nutr. 16:47.
Choudhary, D., S. Bhattacharyya, and S. Bose (2017). Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J. Diet. Suppl. 14:599-612.
Cowley, E., A. Olenick, K. McNulty, and E. Ross (2021). "Invisible sportswomen": The sex data gap in sport and exercise science research. Women Sport Physical. 29:146-151.
Cristofori, F., V.N. Dargenio, C. Dargenio, V.L. Miniello, M. Barone, and R. Francavilla (2021). Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 12:578386.
CRN (Council for Responsible Nutrition) (2020). Consumer survey on dietary supplements.
Dager, S.R., S.D. Friedman, A. Parow, C. Demopulos, A.L. Stoll, I.K. Lyoo, D.L. Dunner, and P.F. Renshaw (2004). Brain metabolic alterations in medication-free patients with bipolar disorder. Arch. Gen. Psychiatry 61:450-458.
De Guingand, D.L., S.J. Ellery, M.L. Davies-Tuck, and H. Dickinson (2019). Creatine and pregnancy outcomes, a prospective cohort study in low-risk pregnant women: study protocol. BMJ Open, 9: e026756.
Draper, C.F., K. Duisters, B. Weger, A. Chakrabarti, A.C. Harms, L. Brennan, T. Hankemeier, L. Goulet, T. Konz, F.P. Martin, S. Moco and J. van der Greef, J. (2018). Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci. Rep. 8:14568.
Edogawa, S., S.A. Peters, G.D. Jenkins, S.V. Gurunathan, W.J. Sundt, S. Johnson, R.J. Lennon R.B. Dyer, M. Camilleri, P.C. Kashyap, G. Farrugia, J. Chen, R,J, Singh, and M. Grover (2018). Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J, fj201800560R.
Everaert, I., A. Mooyaart, A. Baguet, A. Zutinic, H. Baelde, E. Achten, Y. Taes, E. De Heer, and W. Derave (2011). Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids 40:1221-1229.
Filip-Stachnik, A., M. Wilk, M. Krzysztofik, E. Lulinska, J.J. Tufano, A. Zajac, P. Stastny and J. Del Coso (2021). The effects of different doses of caffeine on maximal strength and strength-endurance in women habituated to caffeine. J. Int. Soc. Sports Nutr. 18:25.
Forbes, S.P.A., and L.L. Spriet (2022). Potential effect of beetroot juice supplementation on exercise
economy in well-trained females. Appl. Physiol. Nutr. Metab. 47:106-109.
Freeman, M.P., J.R. Hibbeln, K.L. Wisner, J.M. Davis, D. Mischoulon, M. Peet, P.E. Keck Jr., L.B. Marangell, A.J. Richardson, J. Lake, and A.L. Stoll (2006). Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry, 67:1954-1967.
Garrigues, E., P. Janvier, Y. Kherabi, A. Le Bot, A. Hamon, H. Gouze, L. Doucet, S. Berkani, E. Oliosi, E. Mallart, F. Corre, V. Zarrouk, J.D. Moyer, A. Galy, V. Honsel, B. Fantin and Y. Nguyen (2020). Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 81:e4-e6.
Godoy Reys, P.E., and J. Gimenez-Sanchez (2019). Gastrointensitnal illness in endurance sports women: a review. Arch. Med. Deporte 36:238-247.
Gould, L.M., H.E. Cabre, A.N. Gordon, A.T. Hoyle, K.R. Hirsch, A.A. Ferrando, and A.E. Smith-Ryan. Characterizing the effects of the menopause transition on muscle size and quality. Paper presented at the International Society of Sports Nutrition - unpublished observations.
Gould, L.M., A.N. Gordon, H.E. Cabre, A.T. Hoyle, E.D. Ryan, A.C. Hackney, and A.E. Smith-Ryan (2022). Metabolic effects of menopause: a cross-sectional characterization of body composition and exercise metabolism. Menopause 29:377-389.
Greendale, G.A., N.P. Lee, and E.R. Arriola (1999). The menopause. Lancet, 353:571-580.
Greendale, G.A., B. Sternfeld, M. Huang, W. Han, C. Karvonen-Gutierrez, K. Ruppert, J.A, Cauley, J.S. Finkelstein, S.F. Jiang and A.S. Karlamangla (2019). Changes in body composition and weight during the menopause transition. JCI Insight 4:e124865.
Gruber, C.J., W. Tschugguel, C. Schneeberger, and J.C. Huber (2002). Production and actions of estrogens. N. Engl. J. Med. 346:340-352.
Guest, N.S., T.A. VanDusseldorp, M.T. Nelson, J. Grgic, B.J. Schoenfeld, N.D.M. Jenkins, S.M. Arent, J. Antonio, J.R. Stout, E.T. Trexler, A.E. Smith-Ryan, E.R. Goldstein, D.S. Kalman, and B.I. Campbell (2021). International society of sports nutrition position stand: caffeine and exercise performance. J. Int. Soc. Sports Nutr. 18:1.
Harris, R.C., M.J. Tallon, M. Dunnett, L. Boobis, J. Coakley, H.J. Kim, J.L. Fallowfield, C.A. Hill, C. Sale, and J.A. Wise (2006). The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279-289.
Houltham, S.D., and D.S. Rowlands (2014). A snapshot of nitrogen balance in endurance-trained women. Appl. Physiol. Nutr. Metab. 39:219-225.
Hunt, J.R. (2003). Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 78(3 Suppl):633S-639S.
Hunter, S.K. (2014). Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol. 210:768-789.
IOM (Institute of Medicine) (2005). Dietary reference intakes for energy, carbohdyrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington DC: The National Academies Press.
Jeukendrup, A.E. (2004). Carbohydrate intake during exercise and performance. Nutrition 20:669-677.
Jones, A.M., S.J. Bailey, and A. Vanhatalo (2012). Dietary nitrate and O2 consumption during exercise. Med. Sport Sci. 59:29-35.
Katsanos, C.S., H. Kobayashi, M. Sheffield-Moore, A. Aarsland, and R.R. Wolfe (2005). Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 82:1065-1073.
Kavanaugh, M.L., and J. Jerman (2018). Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 97:14-21.
Kerksick, C.M., C.D. Wilborn, M.D. Roberts, A. Smith-Ryan, S.M. Kleiner, R. Jager, R. Collins, M. Cooke, J.N. Davis, E. Galvan, M. Greenwood, L.M. Lowery, R. Wildman, J. Antonio, and R.B. Kreider (2018). ISSN exercise & sports nutrition review update: research & recommendations. J. Int. Soc. Sports Nutr. 15:38.
Kim, Y.S., T. Unno, B.Y. Kim, and M.S. Park (2020). Sex differences in gut microbiota. World J. Mens Health 38:48-60.
Kisters, K., L. Kisters, T. Werner, A. Deutsch, T. Westhoff, and U. Grober (2020). Increased serum vitamin D concentration under oral magnesium therapy in elderly hypertensives. Magnes. Res. 33:131-132.
Kriengsinyos, W., L.J. Wykes, L.A. Goonewardene, R.O. Ball, and P.B. Pencharz (2004). Phase of menstrual cycle affects lysine requirement in healthy women. Am. J. Physiol. 287:E489-E496.
Kuhman, D.J., K.J. Joyner, and R.J. Bloomer (2015). Cognitive performance and mood following ingestion of a theacrine-containing dietary supplement, caffeine, or placebo by young men and women. Nutrients 7:9618-9632.
Lane, J.D., J.F. Steege, S.L. Rupp, and C.M. Kuhn (1992). Menstrual cycle effects on caffeine elimination in the human female. Eur. J. Clin. Pharmacol. 43:543-546.
Layman, D.K., R.A. Boileau, D.J. Erickson, J.E. Painter, H. Shiue, C. Sather, and D.D. Christou (2003). A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr. 133:411-417.
Lockwood, C.M., J.R. Moon, S.E. Tobkin, A.A. Walter, A.E. Smith, V.J. Dalbo, J.T. Cramer, and J.R. Stout (2008). Minimal nutrition intervention with high-protein/low-carbohydrate and low-fat, nutrient-dense food supplement improves body composition and exercise benefits in overweight adults: A randomized controlled trial. Nutr. Metab. 5:11.
Lovejoy, J.C., C.M. Champagne, L. de Jonge, H. Xie, and S.R. Smith (2008). Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 32:949-958.
McGlade, E., A. Locatelli, J. Hardy, T. Kamiya, M. Morita, K. Morishita, Y. Sugimora, and D. Yurgelun-Todd (2012). Improved attentional performance following citicoline administration in healthy adult women. Food Nutr. Sci. 3:769-773.
Mock, M.G., K.R. Hirsch, M.N.M. Blue, E.T. Trexler, E.J. Roelofs, and A.E. Smith-Ryan (2021). Post-exercise ingestion of low or high molecular weight glucose polymer solution does not improve cycle performance in female athletes. J. Strength Cond. Res. 35:124-131.
Moore, D.R., J. Sygo, and J.P. Morton (2022). Fuelling the female athlete: Carbohydrate and protein recommendations. Eur. J. Sport Sci. 22:684-696.
Ormsbee, M.J., C.W. Bach, and D.A. Baur (2014). Pre-exercise nutrition: the role of macronutrients, modified starches and supplements on metabolism and endurance performance. Nutrients 6:1782-1808.
Parker, B.A., S.L. Smithmyer, J.A. Pelberg, A.D. Mishkin, and D.N. Proctor (2008). Sex-specific influence of aging on exercising leg blood flow. J. Appl. Physiol. 104:655-664.
Phillips, S.M., D.R. Moore, and J.E. Tang (2007). A critical examination of dietary protein requirements, benefits, and excesses in athletes. Int. J. Sport Nutr. Exerc. Metab. 17 (Suppl):S58-S76.
Porri, D., H.K. Biesalski, A. Limitone, L. Bertuzzo, and H. Cena (2021). Effect of magnesium supplemetnation on women's health and well-being. NFS J. 23:30-36.
Robinson, J.G., N. Ijioma, and W. Harris (2010). Omega-3 fatty acids and cognitive function in women. Womens Health 6:119-134.
Sim, J.J., P.T. Lac, I.L. Liu, S.O. Meguerditchian, V.A. Kumar, D.A. Kujubu, and S.A. Rasgon (2010). Vitamin D deficiency and anemia: a cross-sectional study. Ann. Hematol. 89:447-452.
Simopoulos, A.P. (2002). Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21:495-505.
Smith-Ryan, A.E., D.H. Fukuda, J.R. Stout, and K.L. Kendall (2014). The influence of beta-alanine supplementation on markers of exercise-induced oxidative stress. Appl. Physiol. Nutr. Metab. 39:38-46.
Smith-Ryan, A.E., H.E. Cabre, J.M. Eckerson, and D.G. Candow (2021). Creatine supplementation in women's health: A lifespan perspective. Nutrients 13:877.
Sobolewski, E., B. Thompson, A. Smith, and E.D. Ryan (2011). The physiological effects of creatine supplementation on hydration: a review. Am. J. Lifestyle Med. 5:320-327.
Stough, C., J. Lloyd, J. Clarke, L.A. Downey, C.W. Hutchison, T. Rodgers, and P.J. Nathan (2001). The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacol. 156:481-484.
Tarnopolsky, M.A. (2008). Sex differences in exercise metabolism and the role of 17-beta estradiol. Med. Sci. Sports Exerc. 40:648-654.
ten Haaf, D.S., M.P. van der Worp, H.M. Groenewoud, S. Leij-Halfwerk, M.W. Nijhuis-van der Sanden, A.L. Verbeek, and J.B. Staal (2014). Nutritional indicators for gastrointestinal symptoms in female runners: the 'Marikenloop study'. BMJ Open 4:e005780.
Trexler, E.T., A.E. Smith-Ryan, J.R. Stout, J. R. Hoffman, C.D. Wilborn, C. Sale, R.B. Kreider, R. Jäger C.P. Earnest, L. Bannock, B. Campbell, D. Kalman, T.N. Ziegenfuss, and J. Antonio (2015). International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 12:30.
Van Pelt, R.E.,K.M. Gavin, and W.M. Kohrt (2015). Regulation of body composition and bioenergetics by estrogens. Endocrinol. Metab. Clin. North Am. 44:663-676.
Volpe, S.L. (2013). Magnesium in disease prevention and overall health. Adv. Nutr. 4:378S-383S.
Volz, H.P., G. Hubner, R. Rzanny, G. Rossger, B. Preussler, M. Eichhorn, I. Kreitschmann-Andermahr, W.A. Kaiser and H. Sauer (1998). High-energy phosphates in the frontal lobe correlate with Wisconsin Card Sort Test performance in controls, not in schizophrenics: a 31phosphorus magnetic resonance spectroscopic and neuropsychological investigation. Schizophr. Res. 31:37-47.
Wickham, K.A., and L.L. Spriet (2019). No longer beeting around the bush: a review of potential sex differences with dietary nitrate supplementation. Appl. Physiol. Nutr. Metab. 44:915-924.
Wickham, K.A., D.G. McCarthy, J.M. Pereira, D.T. Cervone, L.B. Verdijk, L.J.C. van Loon, G.A. Power, and L.L. Spriet (2019). No effect of beetroot juice supplementation on exercise economy and performance in recreationally active females despite increased torque production. Physiol. Rep. 7:e13982.
Wohlgemuth, K.J., L.R. Arieta, G.J. Brewer, A.L. Hoselton, L.M. Gould, and A.E. Smith-Ryan (2021). Sex differences and considerations for female specific nutritional strategies: a narrative review. J. Int. Soc. Sports Nutr. 18:27.
Yang, P.L., M.M. Heitkemper, and K.J. Kamp (2021). Irritable bowel syndrome in midlife women: a narrative review. Womens Midlife Health 7:4.
Yonkers, K.A., P.M. O'Brien, and E. Eriksson (2008). Premenstrual syndrome. Lancet 371:1200-1210.
Yuan, G., L. An, Y. Sun, G. Xu, and P. Du (2018). Improvement of learning and memory induced by cordyceps polypeptide treatment and the underlying mechanism. Evid. Based Complement Alternat. Med, 9419264.
Zhang, M.M., Y. Zou, S.M. Li, L. Wang, Y.H. Sun, L. Shi, L. Lu, Y.P. Bao, and S.X. Li (2020). The efficacy and safety of omega-3 fatty acids on depressive symptoms in perinatal women: a meta-analysis of randomized placebo-controlled trials. Transl. Psychiatry 10:193.









