NUTRITION AND ATHLETE IMMUNE HEALTH: A NEW PERSPECTIVE
Published
October 2019
Author
Neil P. Walsh PhD, FACSM
Topics

KEY POINTS
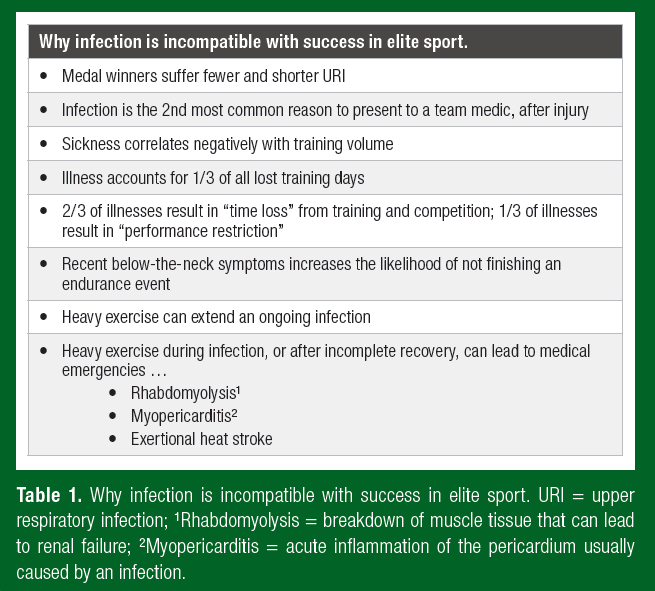
• Sickness absence from training is incompatible with success in elite sport, which demands a consistently high training volume, i.e. the less sick, the more an athlete can train.
• Medal winners at major sporting events, including the Olympics and World Championships, suffer fewer and shorter-lasting respiratory infections than less successful, national level athletes.
• Nutrient availability influences immunity because macro and micronutrients are involved in a multitude of immune processes. Macronutrients are involved in immune cell metabolism and protein synthesis and micronutrients are involved in antioxidant defenses.
• A new paradigm for exercise immunology is presented that considers resistance (the strength of the immune weaponry) and tolerance (the ability to endure microbes and dampen defense activity).
• A contemporary view is that immune resistance is not suppressed in athletes under heavy training, so it is not surprising that nutritional supplements targeted toward improving immune resistance show limited benefits to reduce the infection burden in athletes — ‘if it ain’t broke, don’t fix it!’
• This new paradigm of resistance and tolerance helps to explain why nutritional supplements with tolerogenic effects (e.g., probiotics, vitamin C and vitamin D) are the new targets, as these may reduce the infection burden in athletes.
INTRODUCTION
The aim of this sports science exchange article is to provide a new theoretical perspective to improve our understanding of how nutrition may influence athlete immune health. To set the scene, recent advancements in our understanding of the infection burden in athletes and the prominent infection risk factors will be discussed and scrutinized. As will be the overly simplistic and long-standing view held by many that nutritional supplements should be targeted toward countering the apparently weakened immune weaponry (termed resistance) in otherwise healthy elite athletes. A new paradigm for exercise immunology, recently adopted in human immunology from ecological immunology, will be offered that considers the beneficial tolerogenic interactions (tolerance refers to the ability to endure microbes) between pathogens and the immune system. Looking through this new lens provides a much clearer picture with regard to the rather conflicting and often disappointing findings of studies investigating nutritional supplements and athlete immune health. This new theoretical perspective provides a framework for research on targeted tolerogenic nutritional supplements to reduce the burden of infection in elite athletes.
INFECTIONS POSE A SERIOUS PROBLEM FOR ATHLETES
An upper respiratory infection (URI), such as a common cold, might only present an unwelcome nuisance for many of us. However, URI and other infections such as those that affect the gastrointestinal system may limit an elite athlete’s availability to train and take part in a major competition. After injury, illness (primarily respiratory but also gastrointestinal) was the second most common reason for an elite athlete to seek medical attention either during training or when competing at the summer or winter Olympic Games. In a three-year surveillance study of 322 Olympic athletes, ~70% of illnesses recorded by medical staff resulted in “time loss” (complete absence) from training and competition and the remaining illnesses resulted in “performance restriction” (e.g., reduced volume and/or intensity of training) (Palmer- Green et al., 2013). Clearly, sickness absence from training is incompatible with success in elite sport which demands a consistently high training volume (Table 1). In accordance with this logic, the empirical evidence shows that medal winners at major sporting events, including the Olympics and World Championships, suffer fewer URI and shorter-lasting URI than less successful, national level athletes (Hellard et al., 2015; Svendsen et al., 2016).
Risk Factors for Infection and Lowered Immunity in Athletes
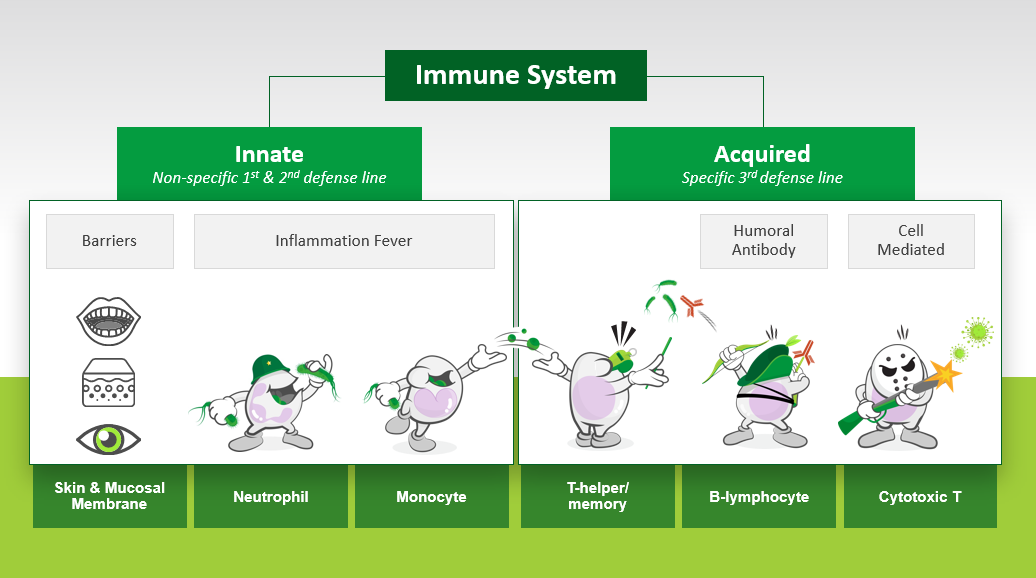
Only very recently has research begun to scratch the surface regarding the prominent risk factors for infection in elite athletes (Table 2). Central to the doctrine of early exercise immunology was the concept that heavy exercise temporarily decreases immunity, providing an “open window” for URI and other infections. Periods of overreaching and longer-term maladaptation (coined overtraining) were also associated with neuroendocrine modulation, decreased immunity and increased URI. These findings supported the prevailing notion of the time that accumulated training stress compromised immune health and increased infection risk. As such, for many years exercise immunologists broadly accepted and focused their research efforts toward countering heavy exercise as a prominent risk factor for URI in athletes. In short, both innate and acquired immunity (Figure 1) are often observed to decrease transiently during the recovery period after prolonged heavy exertion; typically of the order 15%–70% (Walsh, 2018). But whether these transient changes in immunity with acute heavy exercise and intensified training are sufficient to increase URI susceptibility in accordance with the “open window” theory has been in doubt for some time (Ekblom et al., 2006). Findings on URI at the 2000 Stockholm marathon provided the first serious challenge to the “open window” theory by showing no increase in URI symptoms post-race. This contrasted earlier findings showing increased URI after endurance races (Peters & Bateman, 1983). In addition, Ekblom et al. (2006) observations supported the idea that pre-race URI symptoms may have accounted for reports of increased URI after endurance events.
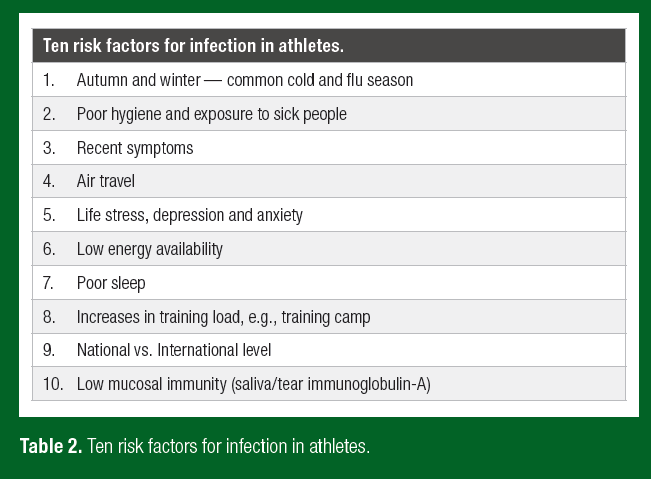
Recent research highlights prominent risk factors for infection in elite athletes and military personnel are broadly similar to those in the wider population (Table 2). Risk factors include, wintertime (common cold and influenza season), high levels of psychological stress, anxiety and depression, poor sleep, and long-haul travel (Drew et al., 2017; Hellard et al., 2015; Svendsen et al., 2016; Wentz et al., 2018). By contrast, increases in training load resulted in relatively small increases in URI and gastrointestinal infection incidence in one study in elite swimmers (Hellard et al., 2015) and no change in infection incidence in another study in elite cross-country skiers (Svendsen et al., 2016). Low energy availability has recently been associated with increased illness symptoms in elite female athletes (Drew et al., 2017). Besides the obvious limitation that this observation was restricted to females, the authors recognized the need for studies to directly assess energy availability (they used the LEAF questionnaire) and perform measures of immunity and pathology, the latter to confirm infection. It is conceivable that poor mental health (e.g., stress, anxiety and depression), highly prevalent in female athletes with low energy availability, also plays a role in the increased URI reports. Psychological stress, anxiety and depression have a well-known and marked influence on immunity and infection resistance (Cohen et al., 1991).
HOW DOES NUTRITION INFLUENCE IMMUNITY AND INFECTION?
The immune system’s ability to clear viruses, bacteria and other pathogens, termed resistance, is dependent upon an adequate supply of energy from important fuel sources, including glucose, amino acids and fatty acids. In addition to fuel requirements, cell proliferation requires nucleotides for DNA and RNA synthesis and amino acids for protein synthesis. An adequate supply of amino acids is also required for the production of proteins such as immunoglobulins, cytokines and acute-phase proteins. The influence of severe restriction of all nutrients (Marasmus) and protein-energy malnutrition (Kwashiorkor) on immunity and infection-related mortality in developing countries is well documented (Woodward, 1998). Severe energy restriction may also influence immunity via activation of the hypothalamic-pituitary-adrenal axis and increases in stress hormones as cortisol, for example, is widely acknowledged to have anti-inflammatory effects. Micronutrients also play important roles in nucleotide and nucleic acid synthesis (e.g., iron, zinc and magnesium) and antioxidant defenses that limit tissue damage (e.g., vitamins C and E). Antioxidant availability (e.g., vitamin C) may be particularly important during heavy exertion or infection when oxidative stress increases. Some micronutrients can directly influence immune cell functions by regulating gene expression (e.g., vitamin D).
There are other ways in which nutrition may affect immunity and infection; for example, prebiotics and probiotics may influence immunity indirectly by modifying the gut microbiota, and elemental zinc in oral lozenges may directly inhibit viral activity in the oropharyngeal region, with therapeutic benefits for URI. Calder (2013) highlighted the bidirectional link between nutrition, immunity and infection. On the one hand, malnutrition has a well-described negative influence on immunity and resistance to infection, but on the other, the widely reported increase in energy requirements during infection paradoxically coincides with reduced appetite (anorexia) and nutrient malabsorption; hitherto, a poorly described phenomenon. Recent research sheds some light on this paradox by showing that reduced appetite improves immune tolerance and survival during bacterial infection (starve a fever …) yet potentiates the progression and lethality of viral infection (… feed a cold) (Wang et al., 2016).
A NEW THEORETICAL PERSPECTIVE ON NUTRITION AND ATHLETE IMMUNE HEALTH
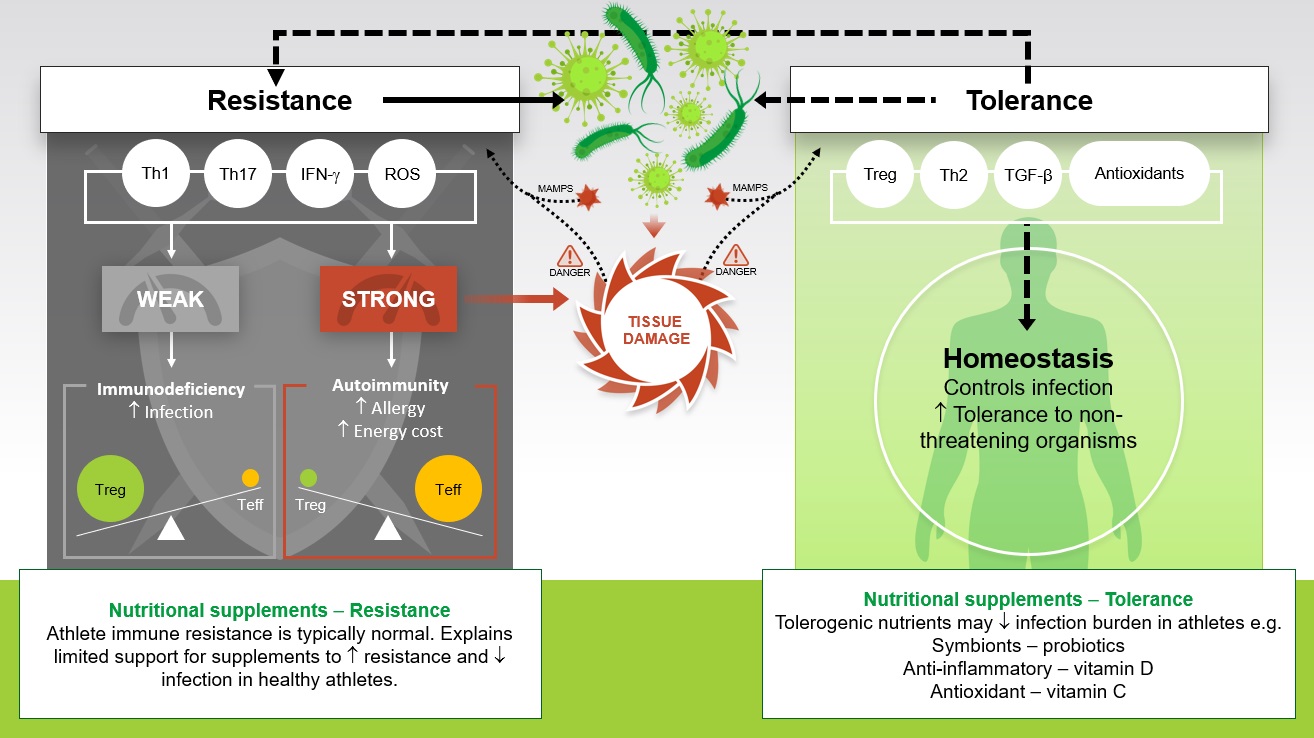
Traditionally, immunologists have focused their efforts on understanding the immune weaponry at our disposal in the fight against infectious pathogens (termed resistance). Ecological immunologists prefer a model describing not only resistance but also tolerance, defined as the ability to endure a microbe. Ayres and Schneider (2012) elegantly described a paradigm using these concepts of resistance and tolerance to better understand human-pathogen interactions. Using a castle metaphor, they describe the inhabitants of the fortress performing various tasks including repairing the walls, raising offspring and distributing food. At the same time, the inhabitants must decide whether a battle is worth fighting and the appropriate weapons to use, the immune equivalent of “choosing your battles wisely”. Key to effective tolerance is a proportionate immune response: an overly exuberant immune response can cause excessive tissue damage and unnecessarily allocate energy resources away from vital functions and vice versa, a weak immune response increases susceptibility to damage from the pathogen (Figure 2). Reactive oxygen species (ROS) play an important role in host defense against infection, but increased oxidative stress during an immune reponse can result in collateral tissue damage, placing an increased demand on antioxidant scavenging during infection. Seminal work in bumblebees has demonstrated the cost of full-blown immune activation for host survival as starvation significantly decreased survival time in immune-activated compared with immunenaïve bumblebees. Given the tissue damage and increased energy cost during a full-blown immune response, the immune system has likely evolved to control persistent infection at a non-damaging level and exhibit tolerance to non-threatening organisms (Figure 2). A prime example is the mutualistic bacteria that reside in the gut; the immune system does not raise a pathogenic response to obliterate the grams of lipopolysaccharide in the intestinal lumen. Homeostasis is achieved by an appropriate balance of resistance and tolerance that allows us to fight infection, where the signals indicate this is necessary, yet maintain a healthy relationship with the mutualistic bacteria in our gut.
This new theoretical perspective may improve our understanding of how sick we will become when we have an infection (in terms of severity and duration), and more clearly elucidate a role for nutrition, particularly in terms of tolerance (Figure 2). Of course, it stands to reason that a frank deficiency of a nutrient required for proper immune function will decrease immune resistance and increase susceptibility to infection. Examples include the well-known influence of dietary protein deficiency on host defense and evidence that a frank deficiency in zinc decreases immunity (Calder, 2013). But growing evidence indicates that for some nutrients there are times when intake above recommended levels may have beneficial effects on immunity, likely by optimizing the delicate balance between resistance and tolerance. Looking through this new lens, illustrated in Figure 2, brings into sharp focus the previously rather mixed picture presented by studies investigating nutritional supplements and athlete immune health. For example, this model helps to explain why nutritional supplements with tolerogenic effects may reduce the burden of infection in otherwise healthy athletes (e.g., reduced severity and duration). Clearly, it’s no longer sufficient to ask only if a nutritional intervention will stop the athlete from getting sick, perhaps it’s more pertinent to ask: Will the nutritional intervention reduce how sick the athlete will get?
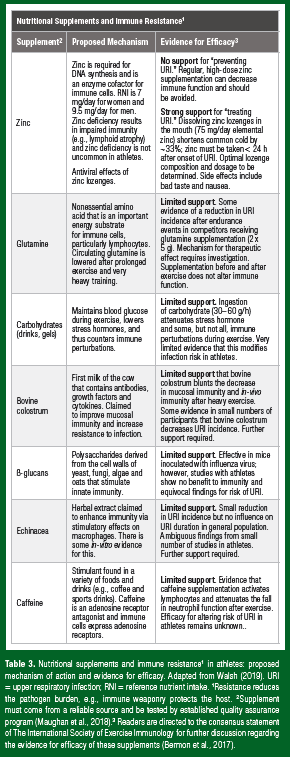
Nutritional Supplements for Immune Resistance: If it ain’t broke, don’t fix it!
As logic would dictate, support for nutritional supplements to improve immune resistance (and thus decrease pathogen burden) comes largely from studies in those with impaired immunity, such as the frail elderly and clinical patients, particularly in those with poor nutritional status (Bermon et al., 2017). Over the last 25 years or so, exercise immunologists have actively researched nutritional supplements to improve immune resistance in athletes (Table 3). For much of this period, there was a broad acceptance among exercise immunologists that immunity was impaired in athletes under heavy training, prompting the search for nutritional countermeasures. A more contemporary view is that the evidence supporting immunosuppression in athletes is lacking. So, it is not surprising that supplements targeted toward immune resistance show limited benefits for athlete immunity and host defense: the phrase “if it ain’t broke, don’t fix it” comes to mind (Table 3). One exception is the therapeutic effect of zinc lozenges for treating the common cold. A recent meta-analysis showed that dissolving zinc lozenges in the mouth (75 mg/day elemental zinc) reduced URI duration by ~3 days (33%) when taken < 24 h after the onset of symptoms, and for the duration of the illness (Hemila, 2017). The author points out that the optimal zinc lozenge dosage and composition need to be determined, as many over-thecounter lozenges contain too little zinc or contain substances that bind zinc. Although the exact mechanism(s) require elucidation, zinc may act as an antiviral agent by increasing interferon gamma and decreasing the docking of common cold viruses with binding sites. The therapeutic effects of zinc lozenges for treating URI have also been ascribed to antioxidant and anti-inflammatory properties of elemental zinc in the lozenge and as such, zinc lozenges may also have tolerogenic effects on immunity.
Tolerogenic Nutritional Supplements: The New Targets
Tolerance in this model dampens defense activity, yet effectively controls infection at a non-damaging level and it also facilitates homeostatic regulation of beneficial intestinal microbial communities (Figure 2). Looking through this lens, it is easy to see why studies involving nutritional supplements with tolerogenic properties have yielded some positive effects for reducing the burden of infection in otherwise healthy athletes (Table 4). Probiotics (and prebiotics) may have tolerogenic effects by influencing intestinal microbial communities and the common mucosal immune system; the antioxidant effects of vitamin C and the antiinflammatory effects of vitamin D may improve tolerance, mitigating against excessive tissue damage during infection. As mentioned previously, the therapeutic effects of zinc lozenges for treating the common cold, although principally considered to reduce the pathogen burden (improved resistance), may also be attributed to antioxidant and anti-inflammatory (tolerogenic) properties of zinc.
Probiotics
Probiotics are live microorganisms, which when administered regularly and in adequate amounts are thought to confer a health benefit on the host by modulating gut-dwelling bacteria (the microbiota) and immunity. There are various mechanisms by which probiotics are purported to benefit immunity and infection resistance, particularly respiratory and gastrointestinal infections; however, thus far, these have not been clearly elucidated. Probiotics can improve immune resistance by reinforcing the intestinal barrier and competing with pathogens for both attachment to the gut epithelium and for available nutrients. The products of probiotic metabolism (e.g., lactic acid) can also inhibit pathogen growth in the gut. Probiotics are considered to have important mutualistic benefits for immune health that extend beyond the gut, as these interactions between the commensal microbial community and the host immune system occur via the common mucosal immune system. There is now broad agreement that probiotics exert important anti-inflammatory, tolerogenic, effects that maintain homeostasis (Figure 2). For example, probiotics may prevent unnecessary inflammatory responses to harmless foreign substances in the gut.
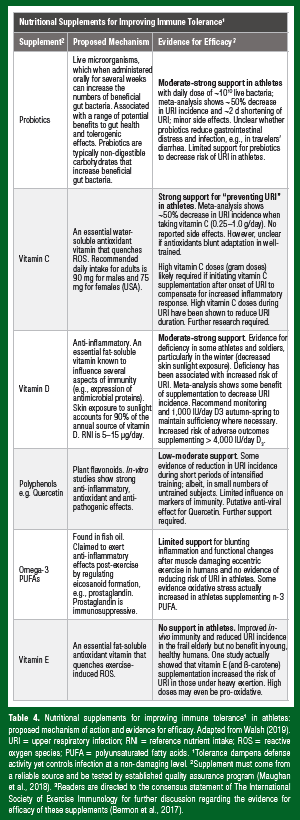
Results from studies investigating the influence of probiotics on athlete immune health are promising and have been extensively reviewed elsewhere (Pyne et al., 2015). One placebo-controlled, cross-over trial in 20 elite distance runners showed that probiotic supplementation (Lactobacillus fermentum) for 28 days reduced the number of days of URI and the severity of URI symptoms (Cox et al., 2010). Another randomized, placebo-controlled trial in 64 university athletes reported a lower incidence of URI during a four-month winter training period in athletes receiving daily probiotic (Lactobacillus casei Shirota) compared with placebo (Gleeson et al., 2011). This study also reported better maintenance of saliva secretory immunoglobulin-A (SIgA) in the probiotic group. Four weeks of supplementation with a multi-species probiotic formulation (Lactobacillus, Bifidobacterium and Streptococcus) reduced markers of gut permeability and symptoms of gastrointestinal discomfort during exercise heat stress (Shing et al., 2014). Whether probiotics and prebiotics can prevent travelers’ diarrhea remains unclear as prophylaxis may be dependent upon the strain of probiotic given. Notwithstanding, results from general population studies show some beneficial effects of probiotics on URI (Table 4). A recent meta-analysis showed that probiotic supplementation reduced the incidence of URI by ~50%, shortened URI duration by ~2 days, reduced antibiotic prescription rates and resulted in only minor side effects (Hao et al., 2015). However, only 12 studies were included in the meta-analysis (n=3,720) and the quality of evidence was rated as low. Limitations included the relatively small sample sizes, poor controls and unclear procedures for randomization. Although the available evidence supporting probiotics to reduce the infection burden in athletes is by no means definitive, studies to date indicate some benefit with little evidence of harm. Athletes might therefore consider probiotic supplementation particularly during periods of increased URI risk, as in the weeks before and during foreign travel (Pyne et al., 2015).
Vitamin C
Vitamin C (ascorbic acid) is a major water-soluble antioxidant that is effective as a scavenger of ROS in both intracellular and extracellular fluids. Good sources of vitamin C include fruit and vegetables and the recommended daily dietary intake for adults is 90 mg for males and 75 mg for females (USA). Vitamin C is found in high concentration in leukocytes, but the level falls dramatically during a common cold when oxidative stress increases. As such, there is a sound scientific basis for vitamin C supplementation to improve tolerance by mitigating against excessive tissue damage during infection (Ayres & Schneider, 2012). There is also a strong rationale for anticipating benefits to reduce URI in athletes who experience increased oxidative stress during heavy exercise. A meta-analysis examined the evidence that daily doses of vitamin C of more than 200 mg have prophylactic and therapeutic effects for the common cold (Hemila & Chalker, 2013). In a subgroup of five placebocontrolled trials in heavy exercisers (n=598), including marathon runners, skiers and soldiers, vitamin C (0.25–1.0 g/day) decreased URI incidence by 52%. For example, in a double-blind, placebo-controlled design, Peters et al. (1993) showed that 600 mg/day of vitamin C for three weeks prior to a 90 km ultramarathon reduced the incidence of URI symptoms in the two-week post-race period (33% vs. 68% in age- and sex-matched control runners). Whether the observed benefit of vitamin C for preventing URI symptoms in those under heavy exertion represents a real decrease in respiratory viral infection is an important avenue for inquiry. The rather high URI symptom incidence in this study (68% in placebo) and the observed benefit of vitamin C might relate to exercise induced bronchoconstriction caused by airway inflammation and injury, which is common during heavy exercise. Regardless of the mechanism, there are clear benefits of vitamin C supplementation (0.25–1.0 g/day) to reduce URI symptoms in athletes (Table 4).
Determining whether initiating vitamin C supplementation after the onset of URI has therapeutic effects is complicated by methodological differences between studies, including differences in the timing of initiating vitamin C supplementation and in the duration and dosage of supplementation (Table 4). Hemila and Chalker (2013) suggest that higher daily vitamin C doses may be required for treating URI and that future therapeutic trials in adults should use doses that exceed 8 g/day vitamin C. Another area of uncertainty is whether regular high-dose vitamin C supplementation (1 g/day) blunts some of the adaptations to endurance training. The authors of one study caution against high-dose antioxidant supplementation during endurance training to avoid blunting cellular adaptations (Paulsen et al., 2014). But whether high-dose antioxidant supplementation blunts training adaptations in highly trained athletes remains unclear. As vitamin C supplementation (0.25–1.0 g/day) is cheap, safe and can prevent URI symptoms in those under heavy exertion, athletes should consider vitamin C supplementation during periods of heightened infection risk, e.g., foreign travel for important competition.
Vitamin D
In 1981, the British general practitioner and celebrated epidemiologist, R. Edgar Hope-Simpson was the first to hypothesize that respiratory viral infections (e.g., epidemic influenza) have a “seasonal stimulus” intimately associated with solar radiation. The nature of this “seasonal stimulus” remained undiscovered until the important immunomodulatory effects of the sunlight-dependent secosteroid vitamin D were fully recognized (Berry et al., 2011). Vitamin D production as a result of sunlight ultraviolet (UV)B radiation penetrating the skin typically provides 80%–100% of the body’s vitamin D requirements, with a small amount typically coming from the diet (good sources include oily fish and egg yolks). The recommended daily dietary intake of vitamin D for adults (5 μg or 200 IU in the European Union and 15 μg or 600 IU in the USA) assumes that no synthesis occurs and all of a person’s vitamin D is from food intake, although that will rarely occur in practice. It is now clear that vitamin D has important roles beyond its well-known effects on calcium and bone homeostasis. Immune cells express the vitamin D receptor, including antigen presenting cells, T cells and B cells, and these cells are all capable of synthesizing the biologically active vitamin D metabolite, 1, 25 hydroxy vitamin D. It is widely accepted that vitamin D plays an important role in enhancing innate immunity via the induction of antimicrobial proteins, yet many of the actions of vitamin D on acquired immunity are anti-inflammatory in nature. Tolerogenic effects of vitamin D (Figure 2) prevent overly exuberant immune responses following T cell activation (e.g., 1, 25 hydroxy vitamin D induces development of regulatory T cells and inhibits production of interferon gamma) (He et al., 2016). There has been growing interest in the benefits of supplementing vitamin D as studies report vitamin D insufficiency (circulating 25(OH)D < 50 nmol/L) in more than half of all athletes and military personnel tested during the winter, when skin sunlight UVB is negligible (Carswell et al., 2018; Close et al., 2013). The overwhelming evidence supports avoiding vitamin D deficiency (circulating 25(OH)D < 30 nmol/L) to maintain immunity and reduce the burden of URI in the general population, athletes and military personnel (He et al., 2016). A recent meta-analysis reported protective effects of oral vitamin D supplementation on respiratory infection (odds ratio 0.88), particularly in those deficient for vitamin D at baseline (odds ratio 0.30) and in those who received oral vitamin D daily or weekly, but not in those receiving one or more large boluses (Martineau et al., 2017). Vitamin D sufficiency can be achieved by safe sunlight exposure in the summer and where screening indicates insufficiency 1,000 IU/day vitamin D3 supplementation in the winter (Table 4) (Carswell et al., 2018).
SUMMARY
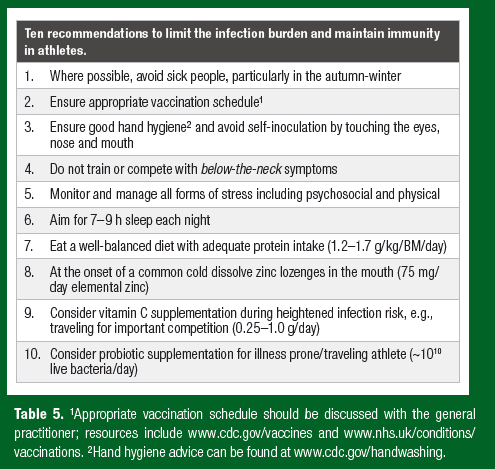
This review provides a new theoretical perspective on how nutrition influences athlete immune health. A paradigm adopted from ecological immunology is presented that includes immune resistance (ability to destroy microbes) and immune tolerance (ability to dampen an immune response and control infection at a non-damaging level). Through this new lens, it is easy to see why studies investigating nutritional supplements targeted at improving immune resistance in athletes show limited benefits: evidence supporting immune suppression in athletes is lacking; that is, if it ain’t broke, don’t fix it! This new perspective sharpens the focus on nutritional supplements with beneficial tolerogenic properties that reduce the infection burden in otherwise healthy athletes, including probiotics, vitamin C and vitamin D. Further research is required demonstrating the benefits of candidate tolerogenic nutritional supplements to reduce the infection burden in athletes, without blunting training adaptations and side effects. When considering nutritional supplementation, athletes must check the supplement comes from a reliable source and is tested by an established quality assurance program (Maughan et al., 2018). Finally, to limit the infection burden and maintain immune health, athletes should follow the practical recommendations in Table 5.
REFERENCES
Ayres, J.S., and D.S. Schneider (2012). Tolerance of infections. Annu. Rev. Immunol. 30:271-294.
Bermon, S., L.M. Castell, P.C. Calder, N.C. Bishop, E. Blomstrand, F.C. Mooren, K. Kruger, A.N.Kavazis, J.C. Quindry, D.S. Senchina, D.C. Nieman, M. Gleeson, D.B. Pyne, C.M. Kitic, G.L.Close, D.E. Larson-Meyer, A. Marcos, S.N. Meydani, D. Wu, N.P. Walsh, and R. Nagatomi(2017). Consensus Statement Immunonutrition and Exercise. Exerc. Immunol. Rev. 23:8-50.
Berry, D.J., K. Hesketh, C. Power, and E. Hypponen (2011). Vitamin D status has a linear associationwith seasonal infections and lung function in British adults. Br. J. Nutr. 106:1433-1440.
Calder, P.C. (2013). Feeding the immune system. Proc. Nutr. Soc. 72:299-309.
Carswell, A.T., S.J. Oliver, L.M. Wentz, D.S. Kashi, R. Roberts, J.C.Y. Tang, R.M. Izard, S. Jackson,D. Allan, L.E. Rhodes, W.D. Fraser, J.P. Greeves, and N.P. Walsh (2018). Influence of vitaminD supplementation by sunlight or oral D3 on exercise performance. Med. Sci. Sports Exerc.50:2555-2564.
Close, G.L., J. Russell, J.N. Cobley, D.J. Owens, G. Wilson, W. Gregson, W.D. Fraser, and J.P.Morton (2013). Assessment of vitamin D concentration in non-supplemented professionalathletes and healthy adults during the winter months in the UK: implications for skeletalmuscle function. J. Sports Sci. 31:344-353.
Cohen, S., D.A. Tyrrell, and A.P. Smith (1991). Psychological stress and susceptibility to thecommon cold. N. Engl. J. Med. 325:606-612.
Cox, A.J., D.B. Pyne, P.U. Saunders, and P.A. Fricker (2010). Oral administration of the probioticLactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. SportsMed. 44:222-226.
Drew, M.K., N. Vlahovich, D. Hughes, R. Appaneal, K. Peterson, L. Burke, B. Lundy, M. Toomey, D.Watts, G. Lovell, S. Praet, S. Halson, C. Colbey, S. Manzanero, M. Welvaert, N. West, D.B.Pyne, and G. Waddington (2017). A multifactorial evaluation of illness risk factors in athletespreparing for the Summer Olympic Games. J. Sci. Med. Sport 20:745-750.
Ekblom, B., O. Ekblom, and C. Malm (2006). Infectious episodes before and after a marathon race.Scand. J. Med. Sci. Sports 16:287-293.
Gleeson, M., N.C. Bishop, M. Oliveira, and P. Tauler (2011). Daily probiotic's (Lactobacillus caseiShirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 21:55-64.
Hao, Q., B.R. Dong, and T. Wu (2015). Probiotics for preventing acute upper respiratory tractinfections. Cochrane Database Syst. Rev.:CD006895.
He, C.S., X.H. Aw Yong, N.P. Walsh, and M. Gleeson (2016). Is there an optimal vitamin D status forimmunity in athletes and military personnel? Exerc. Immunol. Rev. 22:42-64.
Hellard, P., M. Avalos, F. Guimaraes, J.F. Toussaint, and D.B. Pyne (2015). Training-related risk ofcommon illnesses in elite swimmers over a 4-yr period. Med. Sci. Sports Exerc. 47:698-707.
Hemila, H. (2017). Zinc lozenges and the common cold: a meta-analysis comparing zinc acetateand zinc gluconate, and the role of zinc dosage. J.R. Soc. Med. Open 8:2054270417694291.
Hemila, H., and E. Chalker (2013). Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev.:CD000980.
Martineau, A.R., D.A. Jolliffe, R.L. Hooper, L. Greenberg, J.F. Aloia, P. Bergman, G. Dubnov-Raz, S.Esposito, D. Ganmaa, A.A. Ginde, E.C. Goodall, C.C. Grant, C.J. Griffiths, W. Janssens, I.Laaksi, S. Manaseki-Holland, D. Mauger, D.R. Murdoch, R. Neale, J.R. Rees, S. Simpson Jr.,I. Stelmach, G.T. Kumar, M. Urashima, and C.A. Camargo Jr. (2017). Vitamin Dsupplementation to prevent acute respiratory tract infections: systematic review and metaanalysisof individual participant data. Brit. Med. J. 356:i6583.
Maughan, R.J., L.M. Burke, J. Dvorak, D.E. Larson-Meyer, P. Peeling, S.M. Phillips, E.S. Rawson,N.P. Walsh, I. Garthe, H. Geyer, R. Meeusen, L.J.C. van Loon, S.M. Shirreffs, L.L. Spriet, M.Stuart, A. Vernec, K. Currell, V.M. Ali, R.G. Budgett, A. Ljungqvist, M. Mountjoy, Y.P. Pitsiladis,T. Soligard, U. Erdener, and L. Engebretsen (2018). IOC consensus statement: dietarysupplements and the high-performance athlete. Br. J. Sports Med. 52:439-455.
Palmer-Green, D., C. Fuller, R. Jaques, and G. Hunter (2013). The Injury/illness performanceproject (IIPP): a novel epidemiological approach for recording the consequences of sportsinjuries and illnesses. J. Sports Med. 2013:523974.
Paulsen, G., K.T. Cumming, G. Holden, J. Hallen, B.R. Ronnestad, O. Sveen, A. Skaug, I. Paur, N.E.Bastani, H.N. Ostgaard, C. Buer, M. Midttun, F. Freuchen, H. Wiig, E.T. Ulseth, I. Garthe, R.Blomhoff, H.B. Benestad, and T. Raastad (2014). Vitamin C and E supplementation hamperscellular adaptation to endurance training in humans: a double-blind, randomised, controlledtrial. J. Physiol. 592:1887-1901.
Peters, E.M., and E.D. Bateman (1983). Ultramarathon running and upper respiratory tractinfections. An epidemiological survey. S. Afr. Med. J. 64:582-584.
Peters, E.M., J.M. Goetzsche, B. Grobbelaar, and T.D. Noakes (1993). Vitamin C supplementationreduces the incidence of postrace symptoms of upper-respiratory-tract infection inultramarathon runners. Am. J. Clin. Nutr. 57:170-174.
Pyne, D.B., N.P. West, A.J. Cox, and A.W. Cripps (2015). Probiotics supplementation for athletes -clinical and physiological effects. Eur. J. Sport Sci. 15:63-72.
Shing, C.M., J.M. Peake, C.L. Lim, D. Briskey, N.P. Walsh, M.B. Fortes, K.D. Ahuja, and L. Vitetta(2014). Effects of probiotics supplementation on gastrointestinal permeability, inflammationand exercise performance in the heat. Eur. J. Appl. Physiol. 114:93-103.
Svendsen, I.S., I.M. Taylor, E. Tonnessen, R. Bahr, and M. Gleeson (2016). Training-related andcompetition-related risk factors for respiratory tract and gastrointestinal infections in elitecross-country skiers. Br. J. Sports Med. 50:809-815.
Walsh, N.P. (2018). Recommendations to maintain immune health in athletes. Eur. J. Sport Sci.18:820-831.
Walsh, N.P. (2019). Nutrition and athlete immune health: new perspectives on an old paradigm.Sports Med. In press.
Wang, A., S.C. Huen, H.H. Luan, S. Yu, C. Zhang, J.D. Gallezot, C.J. Booth, and R. Medzhitov(2016). Opposing effects of fasting metabolism on tissue tolerance in bacterial and viralinflammation. Cell 166:1512-1525 e12.
Wentz, L.M., M.D. Ward, C. Potter, S.J. Oliver, S. Jackson, R.M. Izard, J.P. Greeves, and N.P. Walsh(2018). Increased risk of upper respiratory infection in military recruits who report sleeping less than 6 h per night. Mil. Med. 183:e699-e704.
Woodward, B. (1998). Protein, calories, and immune defenses. Nutr. Rev. 56:S84-S92.








