CHRONO-NUTRITION: IMPLICATIONS FOR ATHLETE HEALTH AND PERFORMANCE
Published
August 2025
Author
Louise Bradshaw, PhD & James A. Betts, PhD
Topics
Recovery , Carbohydrate , Training & Performance , Sports Nutrition , Athlete Health

KEY POINTS
- Chrono-nutrition refers to the timing of nutrient intake within our regular schedule of daily events and therefore relative to underlying rhythms in our physiology.
- Physiological rhythms span a wide range of timescales and can be driven both by core body ‘clocks’ and external factors, such as regular eating and activity/exercise patterns.
- Skeletal muscle is a key connection, linking rhythms in behavior and metabolism.
- Exercise responses and performance vary according to time-of-day and the fed- versus fasted-state.
- Carbohydrate and protein supplementation pre-, during and post-exercise can be optimized by considering nutrient timing.
- A regular schedule of training and nutrition can specifically prepare an athlete to perform at set times and under particular conditions, which may or may not be advantageous.
INTRODUCTION
Time is the most studied variable in all of science, with most experiments including some form of interaction between a treatment of interest relative to the passage of time (e.g., pre-to-post changes in measurements with a placebo versus an intervention). Simultaneously, the independent effects of time per se are often overlooked, which is certainly the case in the fields of both nutrition in general and sports nutrition in particular. Decades of systematic research have informed extensive evidence-based guidelines about which types of nutrients athletes should ingest and the effective dose of each nutrient, yet precisely when these combinations and amounts of nutrients should be ingested has received far less attention. It is well-established, however, that the physiological response to nutritional interventions can be heavily dependent on context, which in turn highlights the importance of nutrient timing (i.e., to intervene when the appropriate context arises).
Nutrient timing in this sense is often described as ‘chrono-nutrition’, which can be defined not only in terms of the absolute timing of nutrient intake (e.g., time-of-day), but also the temporal sequence of when related events occur surrounding nutritional intervention (e.g., recent meals, sleep and activity/exercise), or when they would usually occur. From one perspective, the availability of nutrients relevant to exercise performance can be entirely framed in relation to timing. For example, an athlete who is ~ 5 lbs (2.3 kg) above or below their ideal weight for optimal performance is usually understood in terms of pure energy balance (i.e., they have ingested more or less energy than is required to fuel their training), but could equally be described as the athlete simply having consumed around one-weeks’ worth of energy too early or too late. More commonly, the primary reason that chrono-nutrition is recognized as an important consideration for athletes is that effective physiological function (i.e., performance) requires the coordination of numerous rhythms in metabolism and behavior. This Sports Science Exchange (SSE) article will summarize some of these rhythms with relevance to sports nutrition, before considering how they might be synchronized to support athlete health and performance.
RESEARCH REVIEW
Rhythms in Physiology
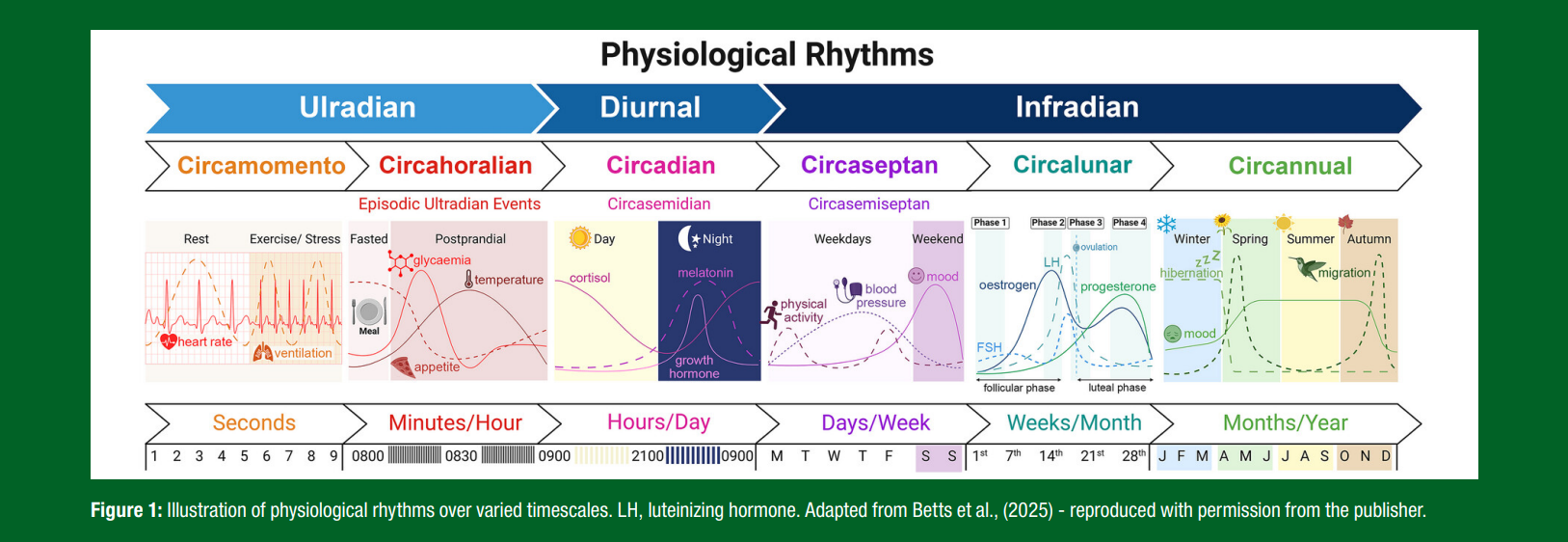
Any repeating temporal pattern can be broadly defined as a rhythm and many physiological processes follow rhythms with some degree of regularity and frequency (Figure 1). These rhythms can be as inherent, persistent and vital to life as the wave-cycle of electrical impulses seen on an electrocardiogram (ECG; which repeat approximately once every second from birth until death), whereas other rhythms can span far longer intervals of weeks or months and may only occur for certain individuals and/or under certain conditions (e.g., the menstrual cycle). The rhythms in our physiology that recur multiple times within one day versus those that recur over multiple days are respectively referred to as ultradian versus infradian rhythms, whereas any repeating cycles that recur almost exactly once every 24-hours are generally referred to as either diurnal or circadian rhythms (Betts et al., 2025)
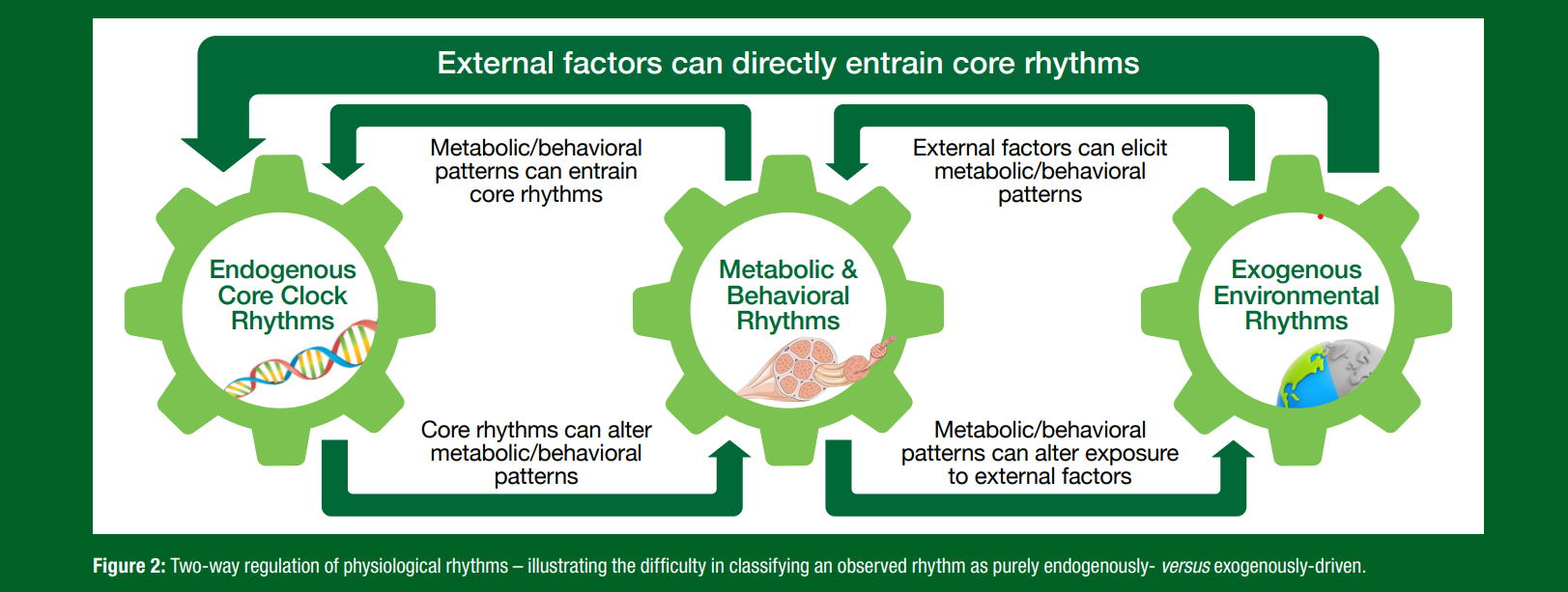
It can also be informative to distinguish between those latter two terms (i.e., diurnal versus circadian) on the basis that diurnal rhythms include the net 24-hour rhythms actually observed on a daily basis, which may be secondary to cyclic patterns in the organism’s environment and/or behaviors (i.e., the apparent rhythmicity in these physiological responses would cease in the absence of those external time-cues). By contrast, circadian rhythms include those 24-hour cycles in our physiology that are primarily endogenously driven and so originate within the organism (such that they would persist for some duration irrespective of external stimuli). This distinction can be useful and certainly has validity, since core circadian rhythms can be attributed to a fundamental mechanism involving negative feedback loops within cells that essentially represent a network of oscillating molecular ‘clocks’ throughout peripheral tissues, all coordinated by a central clock within the suprachiasmatic nucleus (SCN) region of the brain (Albrecht, 2017). However, such a strict distinction between endogenous and exogenous drivers of rhythms can become blurred when realizing that the two are not mutually exclusive. For example, not only are endogenous rhythms often chronically entrained by cyclic external factors, but an organism’s exposure to oscillating exogenous stimuli can in turn be dictated by circadian rhythms in behavior (i.e., an endogenous rhythm in sleep pattern may be both dictated by the daily light-dark cycle, while simultaneously dictating when we are exposed to light on any given day) (Figure 2). Partly for these reasons, reducing physiological rhythms down to a genetic determinist perspective where all mechanisms are molecular (or ‘bottom-up’ – from the SCN) can fail to recognize the more integrative systems-level regulation at play, where ‘top-down’ mechanisms can reveal equally important links between behavior and metabolism (Noble, 2006).
The above thinking is especially relevant to physical exercise because any temporal variance in daily activity patterns can exert profound effects on rhythms in nutrient metabolism. Skeletal muscle is a key tissue of interest in this regard, since it is the sole organ responsible for executing our activity behaviours (i.e., physical movement) and is also the primary site for disposal of ingested macronutrients (e.g. glucose and fatty acids). It is therefore remarkable that human research trials have only relatively recently begun to characterize 24-hour rhythms in human muscle metabolism (for review, see Betts et al., 2025), although these studies to date have generally examined participants under conditions of complete bedrest in order to isolate the effects of diurnal sleep and feeding patterns on diurnal rhythms in metabolism. Further research is therefore needed to similarly study the effects of varied daily patterns of muscle contractile activity in relation to time-of-day and underlying rhythms in metabolism (i.e., chrono-exercise), along with how the relative timing of nutrition may modify the metabolic response to exercise (and vice versa).
Chrono-Exercise
Many of the rhythmic physiological parameters illustrated earlier (e.g., heart rate, glycemia, temperature, blood pressure and endocrine responses (Figure 1)) are also known predictors of human performance. It is therefore understandable why there is variance throughout the day both in tissue-specific metabolic responses to exercise (Sato et al., 2022) and certain aspects of whole-body physical performance. For example, explosive speed (Chtourou et al., 2011) and strength (Douglas et al., 2021) tend to peak later in the day, with less clear evidence for superior endurance in the afternoon/evening (Hill et al., 1988; Knaier et al., 2022). It may therefore be preferable for athletes to compete at those times when performance is greatest (i.e., 16:00- 19:00 h) (Hesketh & Esser, 2024) and there may also be some benefits of scheduling regular training according to the known rhythms of these performance variables, not least because exercise is a potent time-cue that can help entrain the core circadian clock (Martin et al., 2023).
Recent studies have also advanced the understanding regarding how the health effects of regular exercise may be mediated by the time at which training sessions occur. Findings are somewhat equivocal in relation to weight management, generally indicating that reductions in body mass, fat mass and changes in appetite in response to physical training are not consistently different between morning versus evening exercise (Alizadeh et al., 2017; Arciero et al., 2022; Di Blasio et al., 2010; Teo et al., 2021; Willis et al., 2020). Similarly, both fasted and post-prandial glycemia are improved by exercise irrespective of time-of-day but with some indication that evening exercise might be slightly more effective (Kanaley et al., 2024; Kim et al., 2022; Mancilla et al., 2021; Moholdt et al., 2021; Savikj et al., 2019; Teo et al., 2020). While the above effects are describing differences between absolute times of day (i.e., morning versus evening), they are also inherently linked to the relative timing of exercise in relation to other daily events, such as other eating occasions and therefore the nutritional state in which exercise is performed (i.e., low versus high exogenous nutrient availability). It is therefore also relevant to now consider the temporal relationship (i.e., sequence and interval) between exercise and mealtimes.
Exercise-Meal Timing
Manipulating the timing of exercise in relation to exogenous nutrient intake may alter physiological adaptations to endurance training. Completing exercise with limited carbohydrate availability, or in the fasted state, can increase phosphorylation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), which is one of the major signals regulating adaptations to exercise (Mounier et al., 2015). Once activated, AMPK initiates downstream, signalling pathways that directly result in increased mitochondrial biogenesis, and AMPK is also one of the factors related to increased fat oxidation and increased translocation of the glucose transporter type 4 (GLUT4) to the cell membrane (Spaulding & Yan, 2022). Research has shown that training with low carbohydrate availability can increase the expression of genes encoding for key mitochondrial proteins, such as pyruvate dehydrogenase kinase 4, carnitine palmitoyl transferase 1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha and increase cell signalling and oxidative enzyme activity (Hansen et al., 2005; Pilegaard et al., 2005; Steinberg et al., 2006; Yeo et al., 2010). The cumulative effect of multiple bouts of exercise with low carbohydrate availability during a training program could potentially lead to greater adaptations over time, resulting in a greater improvement in performance. However, many training studies that include sessions with low carbohydrate availability, either have not investigated the effect on performance or have reported similar improvements in VO2max (Gejl et al., 2017; Morton et al., 2009; Nybo et al., 2009; Van Proeyen et al., 2011) or time-trial performance (Hulston et al., 2010; Yeo et al., 2008), with only three studies showing improvements in performance under conditions of low carbohydrate availability (Cochran et al., 2015; Marquet et al., 2016a, b).
Peri-Exercise Nutrition
Beyond the above considerations regarding the absolute and relative timing of exercise and nutrition over the course of a day, more specific considerations involve the temporal pattern of nutrient intake surrounding each exercise bout. This includes more conventional recommendations for pre-, during and post-exercise nutrition but in each case taking into account factors concerning nutrient timing, such as when nutritional intervention should start, how frequently or regularly it should be repeated and perhaps also when it should stop. The following sections provide a brief review of some of these aspects of nutrient timing specific to carbohydrate and protein ingestion before, during and after exercise.
Carbohydrate Pre-Exercise. Carbohydrate intake in the hours preceding exercise can provide a final top-up of endogenous carbohydrate stores and provide additional exogenous fuel to muscle ready for the onset of exercise, although there is currently not extensive research on the optimal time of carbohydrate intake pre-exercise (Jeukendrup & Killer, 2010). Carbohydrate intake 30 min, rather than 120 min prior to cycling exercise, can increase time to exhaustion at 90% peak power output (Galloway et al., 2014), whereas carbohydrate intake 15 min, rather than 60 min prior to cycling, did not increase mean power output (Pritchett et al., 2008). Similarly, Mosely et al. (2003) found that 75 g glucose either 15-, 45-, or 75-min pre-exercise resulted in similar performance but with an initial period of hypoglycemia early in exercise when glucose had been ingested 75-min prior. Such ‘rebound’ hypoglycemia is generally a transient response and does not generally impair eventual muscular endurance (Jeukendrup & Killer, 2010), although it is possible that an athlete’s capacity to execute technical skills may be compromised during that initial period of low circulating glucose availability (Afman et al., 2014). Collectively, the balance of available evidence suggests that pre-exercise carbohydrate can elicit greater benefits for performance if ingested anytime within ~1 hour before exercise, but that it may be worth considering any potential metabolic or gastrointestinal disturbances that may result from supplementation so close to exercise.
Carbohydrate During Exercise. The ingestion of carbohydrate during exercise can provide an efficient exogenous source of energy to the contracting muscle, thus maintaining overall carbohydrate availability, reducing perceived exertion and potentially improving physical performance. There are well-developed nutrition guidelines about the quantities and types of carbohydrate that most effectively elicit these outcomes during exercise of varied intensity and duration (Jeukendrup, 2014). In contrast to the many studies comparing doses and types of carbohydrate, there is relatively little research investigating the optimal timing of carbohydrate intake during exercise. Early research investigated different carbohydrate ingestion regimes during cycling across a series of separate studies, revealing similar improvements in time to exhaustion with frequent feeding throughout exercise relative to a single bolus-feeding late in exercise (Coggan & Coyle, 1987; Coyle et al., 1986). In running, however, it was demonstrated that consuming carbohydrate early and frequently throughout exercise resulted in sparing of muscle glycogen and postponed fatigue, compared to when the same quantity of carbohydrate was withheld until late in the exercise bout (Menzies et al., 2020). While theoretically it may therefore be wise to ingest carbohydrate, in small doses and often, during exercise, it may also not be practically advisable or even necessary to do so given that the recommended doses of carbohydrate ingestion are quite large (60-120 g/h-1) thus cannot easily be ingested as a single bolus (i.e., it may be prudent to start early and space-out feedings). Of course, the logistical constraints of a given sporting event may restrict the timing of feedings to certain breaks in play or feeding stations along a course, or to periods of the event when ingestive behaviors are more feasible (such as the cycling phase of a triathlon; Kimber et al., 2002).
Carbohydrate Post-Exercise. Most research into post-exercise carbohydrate ingestion has focused on the replenishment of bodily carbohydrate reserves over the hours following prolonged exercise, particularly the resynthesis of muscle glycogen (Betts & Williams, 2010). Whereas many components of an effective carbohydrate feeding regimen for recovery have been established (such as the different amounts and types of carbohydrate that should be ingested), far fewer studies have examined the various elements of carbohydrate timing (such as how long after exercise carbohydrate feeding should start, how frequently or regularly repeated feedings should occur and when feeding should be terminated ahead of any repeated exercise bout). Nonetheless, it has long been known that there exists a ‘window of opportunity’, whereby ingesting carbohydrate immediately after exercise. rather than delaying intake. can accelerate muscle glycogen replenishment (Ivy et al., 1988), at least for short-term recovery (Betts & Williams, 2010). This is consistent with the mechanistic understanding that prior muscle contractions can profoundly sensitize skeletal muscle to the effects of insulin on glucose transporters and increase muscle membrane permeability (Goodyear et al., 1990; McConell et al., 2020). In addition, as with many nutritional considerations for post-exercise muscle glycogen resynthesis, the frequency of carbohydrate feeding during recovery appears to become more important as recovery duration becomes shorter. Specifically, multiple small frequent snacks versus fewer large bolus meals appear to elicit similar rates of muscle glycogen storage if an athlete has a whole day to recover (Burke et al., 1996), whereas for more rapid short-term recovery (i.e., ≤ 8 hours) the higher published rates of glycogen resynthesis come from those studies in which carbohydrate was ingested at 15-30 min intervals, rather than every 1-2 hours (Betts & Williams, 2010).
Timing of Protein Intake. Consuming an adequate quantity of protein on a daily basis is essential to balance the increased turnover of bodily tissues during periods of intensified exercise, and thus to facilitate physiological adaptations to training (Phillips & van Loon, 2011). As is the case for carbohydrate intake surrounding exercise, research has generated a relatively advanced understanding of the quantity and types/quality of protein that should be ingested to support various outcomes, yet the timing of protein intake has received less research attention (Phillips, 2011).
Similar to the ‘window of opportunity’ described above that advocates ingestion of carbohydrate early in recovery from glycogen-depleting exercise, there has also been some debate over whether protein should equally be consumed immediately following resistance exercise in the hope of capitalizing on an ‘anabolic window’, when rates of muscle protein synthesis and breakdown might be more effectively accelerated and attenuated, respectively (Betts & Stevenson, 2011). Whilst studies in this area are few and equivocal, it seems fair to conclude that any benefit of consuming protein as soon as possible after exercise is less consistent and profound than the need to consume carbohydrate in that manner, yet the balance of available evidence does, to some extent, favor starting protein feeding earlier in recovery. For example, while muscle protein synthesis in the hours following resistance exercise can be similarly stimulated by ingestion of essential amino acids either 1- or 3- hours following exercise (Rasmussen et al., 2000), feeding casein-derived protein immediately following moderate-intensity cycling rather than delaying ingestion by 3- hours revealed a clear acceleration of the acute muscle protein synthetic response (Levenhagen et al., 2001).
These acute responses have also been shown to culminate in greater chronic adaptations. Esmarck et al. (2001), studied responses to 12 weeks of whole-body resistance training (3 d/week-1) amongst elderly men who were randomly assigned to ingest milk/soy protein, either within 5 min or not until 2- hours following each training session. The authors’ main conclusions were that the quadriceps femoris cross sectional area and mean fiber area were significantly increased from baseline in the immediate-feeding group, whereas there was no significant change from baseline in the delayed-feeding group. These findings were mirrored by the increases in strength measurements from baseline within each group (Esmarck et al., 2001). The mean changes for each separate group reported in this study were numerically very clear but it should be noted that the primary inferences were based upon non-parametric analyses of medians and also made based upon within-group changes from baseline rather than the direct contrast between-groups (Bland & Altman, 2015), which did not show any significant strength-promoting advantage of ingesting protein earlier in recovery.
Accepting that increasing amino acid availability early in recovery may be beneficial, there has naturally been further thought about whether it may be of additional benefit to consume protein even earlier, during or before exercise (van Loon, 2014). Tipton et al. (2007) provided exciting early evidence that supplementing with essential amino acids before exercise stimulated post-exercise protein balance more effectively than when the same supplement was consumed post-exercise. The authors speculated that this finding may be attributable to the rapid increase in systemic amino acid availability for the skeletal muscle at a time when blood flow is high during exercise, based partly on their measured differences in amino acid uptake and the fact that the same response was not apparent when later studying a more slowly absorbed intact whey protein (Tipton et al., 2007). Even aside from this suggestion of a more accentuated post-exercise anabolic response, ingestion of protein before and/or during exercise may have the added potential to provide a head-start by commencing the adaptive response to training during either endurance or resistance-based exercise (Beelen et al., 2008; 2011).
Again, translation of acute protein synthetic responses to whole-body and/or functional endpoints in exercise training studies is understandably complicated since those longer-term outcomes are confounded by numerous real-world variables and so are subject to far greater noise in measurement. For example, Shoenfeld et al. (2013), conducted a meta-analysis of all randomized trials reporting changes in muscle strength and/or hypertrophy over at least 6 weeks of resistance training with ingestion of protein (providing at least 6 g of essential amino acids), either within 1- hour before or after exercise, versus no protein within at least 2- hours of exercise. While total protein intake predicted the extent of muscle hypertrophy, neither muscle growth nor strength varied according to the timing of protein intake (Schoenfeld et al., 2013). The same authors published the results of a primary data collection, specifically designed to test the breadth of the time-window around exercise in which protein should be ingested to promote muscular adaptions to training (Schoenfeld et al., 2017). In this practically relevant work, previously resistance-trained men completed 10 weeks of whole-body resistance training (3 d/week-1) with randomization to ingest 25 g of hydrolysed whey protein isolate immediately before, versus immediately post-exercise. Even considering that the former group received no protein for at least 3- hours following each training session, results were consistent with the results of the aforementioned meta-analysis, with no differences between groups in muscle hypertrophy (based on ultrasound of the biceps/triceps brachii and lateral/medial quadriceps femoris), maximal strength (one repetition maximum for bench press and back squat) or body composition (tissue masses), although effect sizes across both groups for all outcomes were generally small and non-significant (probably because participants were in an already trained-state) (Schoenfeld et al., 2017).
The overall balance of available data therefore indicates that there may be some subtle acute acceleration of adaptive metabolic processes by ingesting protein earlier, closer to or before/during exercise, but that this does not consistently translate into meaningful chronic muscular adaptations to training. This is perhaps understandable given that the sensitizing effects of prior muscle contraction on the myofibrillar protein synthetic response to protein ingestion will persist for at least 24 hours (Burd et al., 2011).
The Circadian Flexibility Hypothesis
Having separately considered various specific aspects of the times at which exercise and nutrition should be scheduled relative to one another, these concepts can now be integrated to consider whether these activity and eating patterns should be aligned with underlying rhythms, and thus consistently adhered to on a regular basis. In terms of competitive performance, as noted earlier (Hesketh & Esser, 2024), it is probably a fair assumption that an athlete would ideally prefer to compete at a time-of-day and in a nutritional-state when the available literature and/ or their personal experience suggests they will be at their peak for key performance parameters (e.g., a personal best in powerlifting is less likely to occur first thing in the morning when an athlete might still be in an overnight-fasted state) (Douglas et al., 2021; Kolnes et al., 2025). In terms of training adaptations, however, it is presently a more questionable assumption that daily exercise bouts should always occur at the set times when the body expects muscle contractions and/or is therefore most able to complete training sessions. Although it may at first seem intuitive that our behaviors should conform to the underlying rhythms in our metabolism, such reasoning fails to account for the remarkable capacity of our physiology to recover, adapt and thrive in the face of highly variable conditions.
We forward an alternative hypothesis that optimal physiological function may in fact require a degree of irregularity in daily behaviors and environmental factors, thus not only exercising the ability of our endogenous timing system to acutely respond to those unanticipated stimuli, but in doing so may also hone that ability to adapt, thus maintaining a degree of what might be referred to as ‘circadian flexibility’. While this idea might seem inconsistent with traditional perspectives in chronobiology and observations that circadian misalignment can cause acute metabolic dysregulation, there are a number of similar counter-intuitive examples in the field of sports nutrition and exercise science. Specifically, it is a widely accepted principle of exercise training that a degree of physiological strain is required to elicit adaptations, hence it is entirely logical to progressively overload the system by continually varying the intensity, duration, volume, and/or type of exercise to drive adaptation. A person comfortably completing their daily run at 10 min/ mile pace may benefit from progressing to run a 9 min/mile, or when the body has become accustomed to doing 8 pull-ups then it may be time to aim for 10, and once familiar with using the shoulder-press machine it may be advisable to occasionally try an Arnold-press using free-weights. Why then should it not equally be that varying the time of exercise would also elicit greater physiological strain and so drive superior adaptations to training?
Even if training quality is acutely compromised and/or muscle function is transiently impaired by this ‘mis-timed’ exercise, there are many occasions where such short-term sacrifices are readily accepted in exchange for enhanced chronic adaptations (e.g., training in a fasted-state, at altitude, in the heat and/or with unaccustomed/eccentric muscle actions resulting in exercise-induced muscle damage) (Baranauskas et al., 2021; Burke & Hawley, 2018; McHugh et al., 1999). It is therefore conceivable that an athlete who has purposely trained at irregular times may acquire a competitive advantage even when they have the opportunity to compete at their preferred time of day. Moreover, it seems more reasonable still to speculate that an athlete who has trained their circadian flexibility in this way would be much better prepared to maintain performance in the more common scenarios when life gets in the way and disrupts their typical living pattern (e.g., unable to sleep the night before a big game, heats and finals at different/unusual times of day, or time-zone transition ahead of international competition). In summary, rather than training the body to respond to exercise and/or nutrition for peak performance at just one specific time of day, it remains to be tested whether a more varied and irregular training schedule can produce athletes who are either already better adapted to perform at any time and/or can adapt more quickly to novel or unexpected conditions.
PRACTICAL APPLICATIONS
- Some aspects of physical performance are at their peak in the afternoon and evening.
- Exercise in the fasted state can impair aspects of performance but potentially enhance adaptive responses to training.
- Pre-exercise carbohydrate supplementation can be more effective if ingested within the hour before exercise.
- Carbohydrate ingestion early, in small doses and often during exercise may more effectively improve performance and avoid possible negative side-effects than fewer, larger feedings.
- Post-exercise carbohydrate ingestion should commence as soon as possible following exercise and be consumed at frequent (15-30 min) intervals to promote short-term recovery.
- Ingesting protein before, during or soon after exercise may more effectively stimulate adaptive metabolic responses to training than withholding protein until several hours following exercise.
SUMMARY
Nutrient timing (chrono-nutrition) has the potential to influence various aspects of physiological function, so can be important both for health and performance. The temporal relationship between nutrition and exercise can alter nutrient availability and metabolism, with nutrient intake profoundly altering the metabolic response to exercise and vice versa. The potential benefits of ingesting carbohydrate and protein before, during and after exercise can depend on the precise patterns of ingestion, such as how early and frequently these nutrients are provided in relation to the exercise bout. Competing at certain times of day may be recommended for peak performance but it remains uncertain whether a program of exercise training and sports nutrition should follow a regular or irregular pattern.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Afman, G., R.M. Garside, N. Dinan, N. Gant, J.A. Betts, and C. Williams (2014). Effect of carbohydrate or sodium bicarbonate ingestion on performance during a validated basketball simulation test. Int. J. Sport Nutr. Exerc. Metab. 24:632-644.
Albrecht, U. (2017). The circadian clock, metabolism and obesity. Obes. Rev. 18(Suppl 1):25-33.
Alizadeh, Z., S. Younespour, M. Rajabian Tabesh, and S, Haghravan (2017). Comparison between the effect of 6 weeks of morning or evening aerobic exercise on appetite and anthropometric indices: a randomized controlled trial. Clin. Obes. 7:157-165.
Arciero, P.J., S.J. Ives, A.E. Mohr, N. Robinson, D. Escudero, J. Robinson, K. Rose, O. Minicucci, G. O'Brien, K. Curran, V.J. Miller, F. He, C. Norton, M. Paul, C. Sheridan, S. Beard, J. Centore, M. Dudar, K. Ehnstrom, D. Hoyte, H. Mak and A. Yarde (2022). Morning exercise reduces abdominal fat and blood pressure in women; Evening exercise increases muscular performance in women and lowers blood pressure in men. Front. Physiol. 13:893783.
Baranauskas, M.N., K. Constantini, H.L. Paris, C.C. Wiggins, Z.J. Schlader, and R.F. Chapman (2021). Heat versus altitude training for endurance performance at sea level. Exerc. Sport Sci. Rev. 49:50-58.
Beelen, M., R. Koopman, A.P. Gijsen, H. Vandereyt, A.K. Kies, H. Kuipers, W.H. Saris, and L.J. van Loon (2008). Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am. J. Physiol. 295:E70-E77.
Beelen, M., A. Zorenc, B. Pennings, J.M. Senden, H. Kuipers, and L.J. van Loon (2011). Impact of protein coingestion on muscle protein synthesis during continuous endurance type exercise. Am. J. Physiol. 300:E945-E954.
Betts, J.A., and C. Williams (2010). Short-term recovery from prolonged exercise: exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med. 40:941-959.
Betts, J.A., and E. Stevenson (2011). Should protein be included in CHO-based sports supplements. Med. Sci. Sports Exerc. 43:1244-1250.
Betts, J.A., K.A. Bowden Davies, H.A. Smith, and J.A. Hawley (2025). Physiological rhythms and metabolic regulation: shining light on skeletal muscle. Exp. Physiol. 110:976-983.
Bland, J.M., and D.G. Altman (2015). Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. Am. J. Clin. Nutr. 102:991-994.
Burd, N.A., D.W. West, D.R. Moore, P.J. Atherton, A.W. Staples, T. Prior, J.E. Tang, M.J. Rennie, S.K. Baker, and S.M. Phillips (2011). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 141:568-573.
Burke, L.M., and J.A. Hawley (2018). Swifter, higher, stronger: What's on the menu? Science 362:781-787.
Burke, L.M., G.R. Collier, P.G. Davis, P.A. Fricker, A.J. Sanigorski, and M. Hargreaves (1996). Muscle glycogen storage after prolonged exercise: effect of the frequency of carbohydrate feedings. Am. J. Clin. Nutr. 64:115-119.
Chtourou, H., N. Zarrouk, A. Chaouachi, M. Dogui, D.G. Behm, K. Chamari, F. Hug, and N. Souissi (2011). Diurnal variation in Wingate-test performance and associated electromyographic parameters. Chronobiol. Int. 28:706-713.
Cochran, A.J., F. Myslik, M.J. MacInnis, M.E. Percival, D. Bishop, M.A. Tarnopolsky, and M.J. Gibala (2015). Manipulating carbohydrate availability between twice-daily sessions of high-intensity interval training over 2 weeks improves time-trial performance. Int. J. Sport Nutr. Exerc. Metab. 25:463-470.
Coggan, A.R., and E.F. Coyle (1987). Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J. Appl. Physiol. 63:2388-2395.
Coyle, E.F., A.R. Coggan, M.K. Hemmert, and J.L. Ivy (1986). Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 61:165- 172.
Di Blasio, A., F. Di Donato, M. Mastrodicasa, N. Fabrizio, D. Di Renzo, G. Napolitano, V. Petrella, S. Gallina, and P. Ripari (2010). Effects of the time of day of walking on dietary behaviour, body composition and aerobic fitness in post-menopausal women. J. Sports Med. Phys. Fit. 50:196-201.
Douglas, C.M., S.J. Hesketh, and K.A. Esser (2021). Time of day and muscle strength: A circadian output? Physiology 36:44-51.
Esmarck, B., J.L. Andersen, S. Olsen, E.A. Richter, M. Mizuno, and M. Kjaer (2001). Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 535:301-311.
Galloway, S.D., M.J. Lott, and L.C. Toulouse (2014). Preexercise carbohydrate feeding and high-intensity exercise capacity: effects of timing of intake and carbohydrate concentration. Int. J. Sport Nutr. Exerc. Metab. 24:258-266.
Gejl, K.D., L.B. Thams, M. Hansen, T. Rokkedal-Lausch, P. Plomgaard, L. Nybo, F.J. Larsen, D.A. Cardinale, K. Jensen, H.C. Holmberg, K. Vissing, and N. Ortenblad (2017). No superior adaptations to carbohydrate periodization in elite endurance athletes. Med. Sci. Sports Exerc. 49:2486-2497.
Goodyear, L.J., M.F. Hirshman, P.A. King, E.D. Horton, C.M. Thompson, and E.S. Horton (1990). Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J. Appl. Physiol. 68:193-198.
Hansen, A.K., C.P. Fischer, P. Plomgaard, J.L. Andersen, B. Saltin, and B.K. Pedersen (2005). Skeletal muscle adaptation: training twice every second day vs. training once daily. J. Appl. Physiol. 98:93-99.
Hesketh, S.J., and K.A. Esser (2024). The clockwork of champions: Influence of circadian biology on exercise performance. Free Radic. Biol, Med. 224:78-87.
Hill, D.W., K.J. Cureton, M.A. Collins, and S.C. Grisham (1988). Diurnal variations in responses to exercise of "morning types" and "evening types". J. Sports Med. Phys. Fit. 28:213-219.
Hulston, C.J., M.C. Venables, C.H. Mann, C. Martin, A. Philp, K. Baar, and A.E. Jeukendrup (2010). Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 42:2046-2055.
Ivy, J.L., A.L. Katz, C.L. Cutler, W.M. Sherman, and E.F. Coyle (1988). Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J. Appl. Physiol. 64:1480-1485.
Jeukendrup, A. (2014). A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Med. 44(Suppl 1): S25-S33.
Jeukendrup, A.E., and S.C. Killer (2010). The myths surrounding pre-exercise carbohydrate feeding. Ann. Nutr. Metab. 57(Suppl 2):18-25.
Kanaley, J.A., J.W. Porter, N.C. Winn, G. Lastra, A. Chockalingam, R.J. Pettit-Mee, G.F. Petroski, C. Cobelli, M. Schiavon, and E.J. Parks (2024). Temporal optimization of exercise to lower fasting glucose levels. J. Physiol. 602:6447-6461.
Kim, H.K., S. Furuhashi, M. Takahashi, H. Chijiki, T. Nanba, T. Inami, Z. Radak, S. Sakamoto, and S. Shibata (2022). Late-afternoon endurance exercise is more effective than morning endurance exercise at improving 24-h glucose and blood lipid levels. Front. Endocrinol. 13:957239.
Kimber, N.E., J.J. Ross, S.L. Mason, and D.B. Speedy (2002). Energy balance during an ironman triathlon in male and female triathletes. Int. J. Sport Nutr. Exerc. Metab. 12:47- 62.
Knaier, R., J. Qian, R. Roth, D. Infanger, T. Notter, W. Wang, C. Cajochen, and F. Scheer (2022). Diurnal variation in maximum endurance and maximum strength performance: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 54:169-180.
Kolnes, K.J., E.T.F. Nilsen, S. Brufladt, A.M. Meadows, P.B. Jeppesen, O. Skattebo, E.I. Johansen, J.B. Birk, K. Hojlund, J. Hingst, B.S. Skalhegg, R. Kjobsted, J.L. Griffin, A.J. Kolnes, S. O'Rahilly, J.F.P. Wojtaszewski, and J. Jensen (2025). Effects of seven days' fasting on physical performance and metabolic adaptation during exercise in humans. Nat. Commun., 16:122.
Levenhagen, D.K., J.D. Gresham, M.G. Carlson, D.J. Maron, M.J. Borel, and P.J. Flakoll (2001). Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am. J. Physiol. 280:E982-E993.
Mancilla, R., B. Brouwers, V.B. Schrauwen-Hinderling, M.K.C. Hesselink, J. Hoeks, and P. Schrauwen (2021). Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep. 8:e14669.
Marquet, L.A., J. Brisswalter, J. Louis, E. Tiollier, L.M. Burke, J.A. Hawley, and C. Hausswirth (2016a). Enhanced endurance performance by periodization of CHO intake: "Sleep low" strategy. Med. Sci. Sports Exerc. 48:663-672.
Marquet, L. A., Hausswirth, C., Molle, O., Hawley, J. A., Burke, L. M., Tiollier, E., & Brisswalter, J. (2016b). Periodization of carbohydrate intake: short-term effect on performance. Nutrients, 8:755.
Martin, R.A., M.R.Viggars, and K.A. Esser (2023). Metabolism and exercise: the skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol. 19:272-284.
McConell, G.K., K.A. Sjoberg, F. Ceutz, L. Gliemann, M. Nyberg, Y. Hellsten, C. Frosig, B. Kiens, J.F.P. Wojtaszewski, and E.A. Richter (2020). Insulin-induced membrane permeability to glucose in human muscles at rest and following exercise. J. Physiol., 598:303-315.
McHugh, M.P., D.A.J. Connolly, R.G. Eston, and G.W. Gleim (1999). Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 27:157-170.
Menzies, C., M. Wood, J. Thomas, A. Hengist, J.P. Walhin, R. Jones, K. Tsintzas, J.T. Gonzalez, and J.A. Betts (2020). Frequent carbohydrate ingestion reduces muscle glycogen depletion and postpones fatigue relative to a single bolus. Int. J. Sport Nutr. Exerc. Metab. 30:203-209.
Moholdt, T., E.B. Parr, B.L. Devlin, J. Debik, G. Giskeodegard, and J.A. Hawley (2021). The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia, 64:2061- 2076.
Morton, J.P., L. Croft, J.D. Bartlett, D.P. Maclaren, T. Reilly, L. Evans, A. McArdle, and B. Drust (2009). Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J. Appl. Physiol. 106:1513-1521.
Moseley, L., G.I. Lancaster, and A.E. Jeukendrup (2003). Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur. J. Appl. Physiol. 88:453-458.
Mounier, R., M. Theret, L. Lantier, M. Foretz, and B. Viollet (2015). Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol. Metab. 26:275-286.
Noble, D. (2006). The Music of LIfe. Oxford University Press.
Nybo, L., K. Pedersen, B. Christensen, P. Aagaard, N. Brandt, and B. Kiens (2009). Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 197:117-127.
Phillips, S.M. (2011). The science of muscle hypertrophy: making dietary protein count. Proc. Nutr. Soc. 70:100-103.
Phillips, S.M., and L.J. van Loon (2011). Dietary protein for athletes: from requirements to optimum adaptation. J. Sports Sci. 29(Suppl 1):S29-S38.
Pilegaard, H., T. Osada, L.T. Andersen, J.W. Helge, B. Saltin, and P.D. Neufer (2005). Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54:1048-1055.
Pritchett, K., P. Bishop, R. Pritchett, M. Kovacs, J.K. Davis, C. Casaru, and M. Green (2008). Effects of timing of pre-exercise nutrient intake on glucose responses and intermittent cycling performance. South Afr. J. Sports Med. 20:86-90.
Rasmussen, B.B., K.D. Tipton, S.M. Miller, S.E. Wolf, and R.R. Wolfe (2000). An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. 88:386-392.
Sato, S., K.A. Dyar, J.T. Treebak, S.L. Jepsen, A.M. Ehrlich, S.P. Ashcroft, K. Trost, T. Kunzke, V.M. Prade, L. Small, A.L. Basse, M. Schonke, S. Chen, M. Samad, P. Baldi, R. Barres, A. Walch, T. Moritz, J.J. Holst, D. Lutter, J.R. Zierath, and P. Sassone-Corsi (2022). Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34:329-345
Savikj, M., B.M. Gabriel, P.S. Alm, J. Smith, K. Caidahl, M. Bjornholm, T. Fritz, A. Krook, J.R. Zierath, and H. Wallberg-Henriksson (2019). Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia 62:233-237.
Schoenfeld, B.J., A.A. Aragon, and J.W. Krieger (2013). The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J. Int. Soc. Sports Nutr. 10:53.
Schoenfeld, B.J., A. Aragon, C. Wilborn, S.L. Urbina, S.E. Hayward, and J. Krieger (2017). Pre- versus post-exercise protein intake has similar effects on muscular adaptations. PeerJ, 5:e2825.
Spaulding, H.R., and Z. Yan (2022). AMPK and the adaptation to exercise. Annu. Rev. Physiol. 84:209-227.
Steinberg, G.R., M.J. Watt, S.L. McGee, S. Chan, M. Hargreaves, M.A. Febbraio, D. Stapleton, and B.E. Kemp (2006). Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl. Physiol. Nutr. Metab. 31:302-312.
Teo, S.Y.M., J.A. Kanaley, K.J. Guelfi, K.J. Marston, and T.J. Fairchild (2020). The effect of exercise timing on glycemic control: A randomized clinical trial. Med. Sci. Sports Exerc. 52:323-334.
Teo, S.Y.M., J.A. Kanaley, K.J. Guelfi, J.A. Dimmock, B. Jackson, and T.J. Fairchild (2021). Effects of diurnal exercise timing on appetite, energy intake and body composition: A parallel randomized trial. Appetite 167:105600.
Tipton, K.D., T.A. Elliott, M.G. Cree, A.A. Aarsland, A.P. Sanford, and R.R. Wolfe (2007). Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. 292:E71-E76.
van Loon, L.J.C. (2014). Is there a need for protein ingestion during exercise? Sports Med. 44:(Suppl. 1):S105-S111.
Van Proeyen, K., K. Szlufcik, H. Nielens, M. Ramaekers, and P. Hespel (2011). Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 110:236-245.
Willis, E.A., S.A. Creasy, J.J. Honas, E.L. Melanson, and J.E. Donnelly (2020). The effects of exercise session timing on weight loss and components of energy balance: midwest exercise trial 2. Int. J. Obes. 44:114-124.
Yeo, W.K., C.D. Paton, A.P. Garnham, L.M. Burke, A.L. Carey, and J.A. Hawley (2008). Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 105:1462-1470.
Yeo, W.K., S.L. McGee, A.L. Carey, C.D. Paton, A.P. Garnham, M. Hargreaves, and J.A. Hawley (2010). Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp. Physiol. 95:351-358.








