Recovery Nutrition for the Basketball Athlete
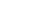
INTRODUCTION
Skill is essential to performance in basketball. But at a certain level, everyone has skill. What sets one skilled athlete apart from another is their strength, speed, and power. Strength, speed and power are dependent on a player’s muscle mass, muscle type (fast vs. slow), ability to send the right signals to the muscle from the brain, and the stiffness of the connective tissue that connects the muscle to the bone. When athletes train, these are the things that they are trying to improve (all, except a player’s muscle type, can be improved with training). Every coach knows that when you train a team, some individuals respond better than others. In part, this is due to genetics. But a large part of the difference can be the result of differences in nutrition. This chapter will introduce simple ways that athletes can use nutrition to improve the response to training. For more information on this topic, please see a more thorough review.12
KEY FINDINGS
MAINTAINING AND GROWING MUSCLE
An athlete’s strength, speed, and power are dependent on their muscle mass, which may be increased through strength training. However, without the proper nutrition, strength training is not enough to increase mass and strength,13 and it is extremely difficult to build strength when also practicing for long hours at a high intensity.7 In fact, it is not uncommon for an athlete to lose weight during a season as a result of the sheer amount of training and competition. In rapidly growing individuals such as teenage basketball players, weight loss can be even more dramatic. Some of the weight lost will be from a decrease in body fat, but it is just as common to lose muscle mass. The goal of recovery nutrition is to help maintain/grow muscle and make sure that any weight lost during the season is fat. The key to this is not only how many calories the athlete takes in, but also the type of food and when they eat it.
An athlete’s muscle mass is determined by the balance between how much muscle protein they make and how much muscle protein they break down. In a fasting athlete, both muscle protein synthesis and breakdown go up following training. The result is that a fasted athlete cannot build muscle mass. The body only starts to build muscle when supplied with protein.15 When an athlete eats protein after training, it increases protein synthesis more than training alone, and proteins rich in essential amino acids prevent some of the rise in protein breakdown.15 The result is a big shift in balance so that athletes can begin to add muscle mass.
Because of the important role of protein in stimulating muscle protein synthesis during recovery, athletes should consume protein within about the first 30 minutes after training. The timing of the protein intake is important for two reasons: 1) blood flow and 2) molecular signaling. If an athlete consumes protein soon after training, the muscles that were just trained will have more blood flow, and therefore more of the protein from the meal will be delivered to the muscles they are training. When the amino acids from the protein meal arrive at the muscle, they turn on signaling processes that activate muscle protein synthesis. The end result is that simply shifting some of your athlete’s protein intake to the period immediately after training will result in more amino acids getting to the muscle and more protein synthesis.
So, it is clear that nutrition in recovery from training can improve muscle growth, but what should athletes be eating? As far as the recovery period, the amino acids are the key. Adding carbohydrates to a recovery drink/meal has no further beneficial effect specifically on muscle protein synthesis or degradation. As far as the amino acids, the focus should be to have all of the essential amino acids and a high amount of the branched chain amino acid leucine. It is also important that the protein is easy to absorb. For example, a steak has all of the essential amino acids but is difficult to absorb. Simply grinding the steak into hamburger makes it easier to absorb and will get more amino acids to the muscle. In a similar way, the two protein components of milk are absorbed at different rates. Casein is slowly digested because it clumps in the acid of the stomach, whereas whey is rapidly absorbed and is richer in leucine than soy-based proteins. The high leucine level triggers muscle protein synthesis, whereas the rest of the essential amino acids are needed to make the new protein. The result is that taking leucine-rich whey protein in recovery from training results in more protein synthesis and muscle growth than either soy or casein.14 The best sources of leucine-rich proteins are milk, eggs, and whey- based recovery products.
The next question is how much leucine-rich protein should athletes consume? There are a number of studies that suggest that an athlete should take 0.25g of protein per kilogram of body weight after training (Figure 1).10 This means that a 175lb (~80kg) athlete would want to get 20g of protein, whereas a smaller, 130lb (~60kg) athlete would want to get 15g of protein. Any more protein intake at one time will not benefit the muscles.
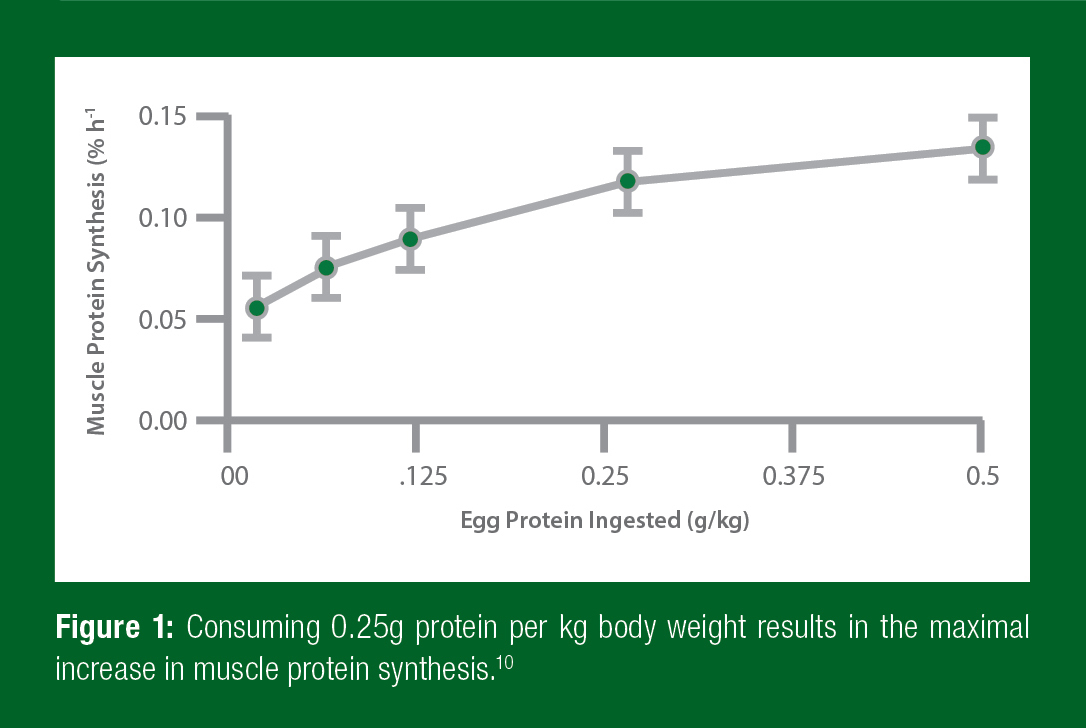
This data suggests that taking 0.25g/kg of a leucine-rich protein within 30 minutes of training will result in the best response within an athlete’s muscles. However, it is important to remember that recovery doesn’t end 30 minutes after training. In fact, after strength training, muscles are more sensitive to protein feeding for at least 24 hours.3 That means that every time athletes eat protein for a full day after training, they make more muscle protein. As a result of this increased sensitivity, it is important to eat that 0.25g/kg amount of protein at meals every 3–4 hours throughout the day. In fact, eating the same total amount of protein in smaller doses more frequently, or bigger doses less often, are not as good at increasing muscle protein synthesis.2
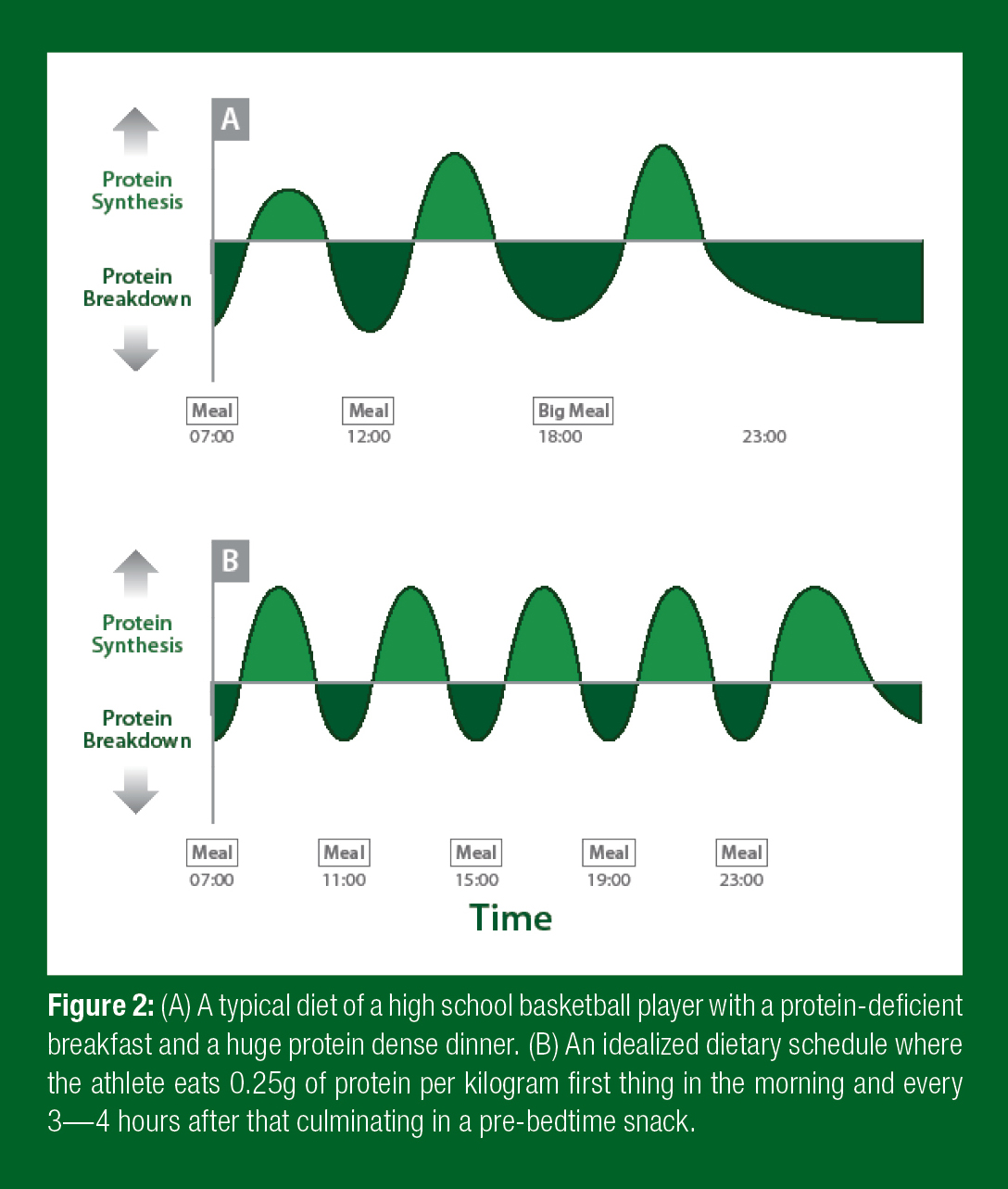
This is in contrast to the habits of high school basketball players, who normally eat a small, protein-deficient breakfast, a moderate-protein lunch, and a large-protein dinner (Figure 2A). Since protein synthesis and degradation are dependent on the presence of amino acids, the result is a net protein breakdown (more dark green areas under the line than green areas above it). If instead an athlete would consume 0.25g/ kg protein first thing in the morning and then every 3—5 hours from there on, they would synthesize more protein than they break down, resulting in a gain in muscle mass (Figure 2B). Taking the same amount of protein right before bed can boost muscle growth even more. Protein before bed delays the sleep (fasting)-induced shift toward negative protein balance, saving muscle from breakdown.
IMPROVING CONNECTIVE TISSUE HEALTH AND FUNCTION
Another component of strength, speed, and power is the stiffness of an athlete’s connective tissue. Connective tissues include not only an athlete’s tendons and ligaments, but also the collagen within a muscle that transfers the force made by the muscle to the tendon and bone. Athletes and their coaches normally only think of connective tissues when they have an injury. A pulled hamstring or a ruptured anterior cruciate ligament (ACL) is failed connective tissue and will need extensive rehabilitation. Young female basketball players are four times more likely to experience this type of injury than their male counterparts1, and therefore the issue of connective tissue health is especially important to women’s basketball coaches. However, beyond health, connective tissue is important in performance. There is a direct relationship between muscle-tendon stiffness and jump height.5 The best way to increase muscle-tendon stiffness and jump performance is to perform rapid plyometric exercises (jumps, sprints, bounds, or playing in actual basketball games). However, even though this is good for performance, greater muscle-tendon stiffness is associated with an increased incidence of muscle injury,9 so exercise caution with a lot of plyometric activities in training. Reversing muscle-tendon stiffness is as easy as doing the same movements slowly and concentrating on the negative or lengthening phase (i.e., the lowering phase of a toe raise).8 Therefore, performing slow movements in training can decrease an athlete’s stiffness (and likelihood for injury).
The issue of connective tissue function is especially important for high school athletes for one other reason: The core of a tendon doesn’t change after the age of 18.6 Therefore, what high school athletes do during this critical window will shape their tendons for the rest of their lives.
So, it is clear that there is a balance between performance and injury when it comes to connective tissue and that fast plyometric movements increase stiffness and slow lengthening movements decrease stiffness. What is less clear is how these processes can be enhanced with nutrition. First, unlike muscle, connective tissue doesn’t have a large blood flow. Instead, tendons and ligaments work more like sponges. When stretched or loaded, fluid is squeezed out, and when relaxed, new fluid is sucked in. This means that nutrients that might improve tendon and ligament function need to be in the blood stream before exercise. Second, there are only a handful of studies in humans that have shown a nutritional intervention that improves connective tissue. One recent study showed that consuming ~10g of whey protein before and after resistance exercise resulted in more hypertrophy of not only the muscle but of the tendon as well.4 The result was an improvement in the rate of force development, in part due to the tendon adaptation. From some basic research, we can suggest some other things that might work, but they have yet to be validated in humans. The most promising nutrients for connective tissue health are vitamin C and proline. In culture, we can make ligaments stronger by adding vitamin C and proline.11 These nutrients are found in gelatin, so we have been advising young athletes and those who are prone to injury to eat 1⁄4–1⁄2 cup of vitamin C- enriched gelatin about 30 minutes before training. However, we still don’t have any scientific evidence that this decreases injury or improves performance.
SUMMARY
Differences in strength, speed, and power differentiate skilled basketball players from the elite. Building strength, speed, and power requires proper training AND nutrition. To increase muscle mass and strength through a basketball season, an athlete should consume 0.25g of leucine-rich protein per kilogram of body weight every 3–4 hours and within about the first 30 minutes after training. Proteins such as milk, whey, eggs, and meat are ideal for this purpose. Not only is muscle important for strength, but we are also learning that connective tissue plays an important role. However, we don’t yet know whether we can improve this with nutrition.
REFERENCES
-
Arendt, E., and R. Dick (1995). Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 23:694- 701.
-
Areta, J.L., L.M. Burke, M.L. Ross, D.M. Camera, D.W. West, E.M. Broad, N.A. Jeacocke, D.R. Moore, T. Stellingwerff, S.M. Phillips, J.A. Hawley, and V.G. Coffey (2013). Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 1;591:2319-2331.
-
Burd, N.A., D.W. West, D.R. Moore, P.J. Atherton, A.W. Staples, T. Prior, J.E. Tang, M.J. Rennie, S.K. Baker, and S.M. Phillips. (2011). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 141:568-573.
-
Farup, J., Rahbek, S.K., Vendelbo, M.H., Matzon, A., Hindhede, J., Bejder, A., Ringgard, S., and Vissing, K. (2013). Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand. J. Med. Sci. Sports. doi: 10.1111/sms.12083. [Epub ahead of print].
-
Foure, A., A. Nordez, M. Guette, and C. Cornu (2009). Effects of plyometric training on passive stiffness of gastrocnemii and the musculo-articular complex of the ankle joint. Scand. J. Med. Sci. Sports. 19:811-818.
-
Heinemeier, K.M., P. Schjerling, J. Heinemeier, S.P. Magnusson, and M. Kjaer (2013). Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEBJ. 27:2074-2079.
-
Hickson, R.C. (1980). Interference of strength development by simultaneously training for strength and endurance. Eur. J. Appl. Physiol. Occup. Physiol. 45:255-263.
-
Mahieu, N.N., P. McNair, A. Cools, C. D’Haen, K. Vandermeulen, and E. Witvrouw (2008). Effect of eccentric training on the plantar flexor muscle-tendon tissue properties. Med. Sci. Sports Exerc. 40:117-123.
-
McHugh, M.P., D.A. Connolly, R. G. Eston, I.J. Kremenic, S.J. Nicholas, and G.W. Gleim (1999). The role of passive muscle stiffness in symptoms of exercise-induced muscle damage. Am. J. Sports Med. 27:594-599.
-
Moore, D.R., M.J. Robinson, J.L. Fry, J.E. Tang, E.I. Glover, S.B. Wilkinson, R. Prior, M.A. Tarnopolsky, and S.M. Phillips (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89:161-168.
-
Paxton, J.Z., L.M. Grover, and K. Baar (2010). Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng. Part A. 16:3515-3525.
-
Phillips, S., K. Baar, and N. Lewis (2011). Nutrition for Weight and Resistance Training. In: S. Lanham-New, S. Stear, S. Shirreffs, and A. Collins (eds) Nutrition Society Textbook on Sport and Exercise Nutrition. Oxford, UK: Wiley-Blackwell. pp. 120-133.
-
Phillips, S.M., K.D. Tipton, A. Aarsland, S.E. Wolf, and R.R. Wolfe (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 273:E99-107.
-
Tang, J.E., D.R. Moore, G.W. Kujbida, M.A. Tarnopolsky, and S.M. Phillips (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 107:987-992.
-
Tipton, K.D., A.A. Ferrando, S.M. Phillips, D. Doyle Jr., and R.R. Wolfe (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 276:E628-634.








