PROTEIN-CONTAINING FOOD IS MORE THAN THE SUM OF ITS CONSTITUENT AMINO ACIDS FOR POST-EXERCISE MUSCLE ANABOLIC POTENTIAL
Published
May 2019
Author
Nicholas A. Burd; Colleen F. McKenna; Sarah K. Skinner; Isabel G. Martinez
Topics

KEY POINTS
- Dietary amino acids are the main drivers of post-exercise muscle protein synthesis (MPS) rates.
- Exercise modifies the handling of dietary protein and how it is used for post-exercise MPS.
- The ingestion of isolated protein supplements results in maximal protein dosing for post-exercise MPS.
- The ingestion of whole foods, which contain a food matrix rich in dietary protein, vitamins, minerals, and other macronutrients, is also a potential strategy to optimize protein in the diet for post-exercise MPS.
- An individualized approach should be used when identifying the most appropriate post-exercise recovery nutrition option for an athlete, and whether to incorporate protein-rich whole foods, supplements, or both.
INTRODUCTION
Exercise and dietary protein (amino acids) ingestion are two of the main anabolic stimuli to increase muscle protein remodeling, primarily by the stimulation of muscle protein synthesis (MPS) rates in healthy adults. When these two anabolic stimuli are combined on a regular basis, through eating regular protein-dense meals and performing exercise training, it ultimately facilitates the adaptive processes that aid in improving exercise performance. A weightlifter is generally monitoring improved performance by strength or power progressions, which is facilitated by both neural and muscular adaptations. Conversely, an endurance athlete is training to improve aerobic capacity and associated adaptations in central and peripheral factors. Regardless of the goal, protein intake is required to maximize the adaptation for all training programs. Immediate post-exercise protein intake is heavily emphasized and encouraged due to the relatively strong additive effect of dietary amino acids on the mechanisms that control MPS at this time (Biolo et al., 1997). However, all meals are relevant and have the potential to maximize the muscle adaptive response by potentiating the feeding stimulation of MPS during a prolonged post-exercise recovery window (Burd et al., 2011).
Current concepts around optimal protein nutrition for athletes to maximize adaptive MPS processes have been based on reductionist approaches, or viewing protein through the lens of its constituent amino acids and even down to the anabolic potency of a single amino acid such as leucine. However, athletes do not consume individual nutrients but instead eat food combinations throughout the day to facilitate optimal recovery. A more holistic approach to protein nutrition guidelines may help ensure replenishment of fuel stores through appropriate carbohydrate and fat intake protocols and perhaps more efficient use of dietary protein-derived amino acids for MPS stimulation during exercise recovery.
PROTEIN QUALITY
Protein quality is often based on the digestibility of a dietary protein source in relation to human essential amino acid requirements. The ingested protein needs to be digested into amino acids and absorbed into the blood in adequate amounts in order to provide a strong anabolic signal to skeletal muscle tissue. In general, plant-based protein sources (e.g., soy, wheat and pea) demonstrate lower digestibility than animal-based protein sources (e.g., milk, beef, pork and fish). However, food processing techniques of plant-based protein sources can improve their digestibility (van Vliet et al., 2015). Certainly, isolated protein powders generally hold a position of prominence among athletes and gym-goers due to their high digestibility and rapid delivery of dietary amino acids into the blood. However, isolated protein powders are not required to elicit a robust rise in the post-exercise MPS response (Burd et al., 2015).
What is noteworthy is that both resistance and endurance exercise induce redistribution of blood flow to peripheral tissues (contracting muscles) and reduce blood flow to the gut. This can potentially impact the absorption profiles of nutrients consumed within a post-exercise recovery beverage or meal. For example, exercise-induced splanchnic hypoperfusion can induce small intestinal injury leading to increases in gastrointestinal (GI) permeability. This compromise in epithelial integrity has been shown to negatively affect intestinal absorption and/or splanchnic sequestration of dietary amino acids (van Wijck et al., 2013), which may subsequently limit the postprandial rise in dietary amino acid availability during post-exercise recovery. In our hands, however, we routinely see > 60% of the dietary amino acids becoming available to the periphery during recovery from exercise in trained adults (Mazzulla et al., 2017; van Vliet et al., 2017). This amount of dietary protein-derived amino acids is very similar to the amount that becomes available in the circulation after eating a meal in the resting state in healthy adults (Mazzulla et al., 2017). Interestingly, exercise-induced increases in GI permeability may ultimately adjust intestinal barrier function allowing more foodborne bioactive peptides to cross in the exercise vs. non-exercise state (JanssenDuijghuijsen et al., 2016). Hence, exercise creates an avenue in the intestinal barrier allowing for small molecules ingested in food to be absorbed that may not otherwise have the opportunity to transfer in the resting state. Finally, repeated bouts of exercise have the potential to modify the complexity and dynamics of microorganisms colonizing in the gut (i.e., gut microbiota). In fact, intestinal microbes can synthesize amino acids with their contribution estimated, in the case of leucine, to be ~20% of the total body input (Raj et al., 2008). It is currently not clear how exercise-induced changes in gut microbiota function and composition would modulate whole-body or muscle protein metabolism. In any case, it is evident that exercise can acutely impact the intestinal tract (van Wijck et al., 2013; JanssenDuijghuijsen et al., 2016) as well as, in the longer term, modulate gut microbial communities and their functional metabolic outputs (Allen et al., 2018). Such effects on the intestinal tract may have a direct impact on how post-exercise dietary amino acids are delivered to skeletal muscle tissue for protein remodeling and repair. Moreover, foods with supporting bioactive peptides may have greater anabolic potential if consumed in the post-exercise state.
“OPTIMAL” PROTEIN REQUIREMENT
Defining the optimal and/or excessive amount of protein in a meal is relevant for an athlete. An excessive amount of protein in a meal has the potential to displace other vital nutrients (An & Burd, 2015) and has implications for financial and environmental strain (Meyer & Reguant-Closa, 2017). With respect to skeletal muscle, an optimal amount of protein in a meal is commonly defined as the amount where MPS is maximally stimulated with minimal amino acid oxidation rates. An excessive amount of protein in a meal is defined as the point where amino acids show an exponential increase in oxidation rates and MPS is not further stimulated in response to increasing amounts of ingested protein. Indeed, this question of optimal protein meal requirements for athletes is most commonly addressed by attempting to define the maximal amount of isolated protein sources to consume in a meal to elicit a robust rise in post-exercise MPS. Further work is required to test strategies to facilitate more optimal use of protein in the diet for post-exercise MPS and repair. In other words, the question that needs to be addressed is: How can we elicit an optimal MPS response without simply eating more and more protein in the diet? One emerging concept is related to using specific foods or food mixtures to potentiate the use of dietary amino acids for post-exercise MPS. Conceptually, this is often referred to as food synergy, or relationships existing between the basic food components such that they provide stronger metabolic effects when combined versus ingested alone (Jacobs et al., 2009).
Nonetheless, based on these maximal guidelines, the amount of protein in a meal to stimulate post-exercise MPS with minimal amino acid oxidation rates is wide-ranging and falls somewhere between 20–40 g of protein per meal for healthy individuals. Indeed, even the ingestion of an “optimal” quantity of protein in a meal to stimulate post-exercise MPS will increase whole-body leucine oxidation rates (Moore et al., 2009a), which may be a result of using isolated protein sources to address this question. For example, our research group has recently shown that it is possible to elicit a robust post-exercise MPS response, without stimulating an increase in whole-body leucine oxidation rates, when using a whole-foods approach (van Vliet et al., 2017).
It is worth noting that these protein meal guidelines to facilitate the post-exercise muscle adaptive response have been developed based on recovery from a weightlifting stimulus. Lifting weights is inherently anabolic, associated with a short duration of contraction and fuel use, and enhances the amino acid sensitivity of skeletal muscle tissue for a prolonged period of time during recovery (Burd et al., 2011). In fact, research has shown that there are redundant methods available via various contraction manipulations (i.e., intensity, volume, etc.) to maximize the dietary amino acid sensitivity of muscle fibers to protein ingestion after a weightlifting workout (Burd et al., 2011). Thus, dietary amino acids in circulation can be preferentially used for the repair and remodeling of muscle proteins after weightlifting to provide the basis for training-induced adaptations, such as hypertrophy. However, endurance exercise-based activities result in more amino acids being used as fuel, and their usage can be enhanced by various factors including, but not limited to, exercise intensity and duration. The amino acids (e.g., leucine) that are oxidized during exercise are irreversibly lost from the body and thus must be replaced via dietary protein. This ultimately places substantial stress on protein nutrition, especially in the immediate post-exercise meal (Mazzulla et al., 2017), to replace both the exercise-induced amino acid oxidative losses and to remodel muscle fibers during recovery from endurance exercise. As a result, more specific protein guidelines incorporating “periodized” nutrition may be necessary to facilitate optimal exercise-adaptive MPS processes during recovery from endurance (Abou Sawan et al., 2018) versus resistance-based activities (Burd et al., 2011), especially in the meal consumed immediately post-exercise.
DIETARY AMINO ACIDS: DIRECT STIMULATORS OF MUSCLE PROTEIN SYNTHESIS
It is evident that eating protein after exercise stimulates post-exercise MPS (Moore et al., 2009b) and that essential amino acids are required for a robust effect (Volpi et al., 2003). Interestingly, the feeding induced potentiation of post-exercise MPS is more apparent after weightlifting (Burd et al., 2011) when compared to endurance exercise (Abou Sawan et al., 2018). As noted above, the lack of additive effect between dietary amino acid availability and endurance exercise on post-exercise MPS may relate to the fact that some of the dietary amino acids need to be aimed at replacing exercise-induced amino acid oxidative loss (Mazzulla et al., 2017), and thus limits the amount of dietary amino acids available for post-exercise MPS and remodeling (Abou Sawan et al., 2018). The amino acid leucine has received considerable attention regarding its contribution to stimulating the post-exercise MPS response. In part, leucine is not only a building block for muscle protein, but also serves as a signaling molecule for MPS in human skeletal muscle tissue. Moreover, the speed and amplitude of the postprandial rise in circulating leucine after the ingestion of isolated protein sources has been shown to modulate the post-exercise MPS response in healthy young adults (Tang et al., 2009).
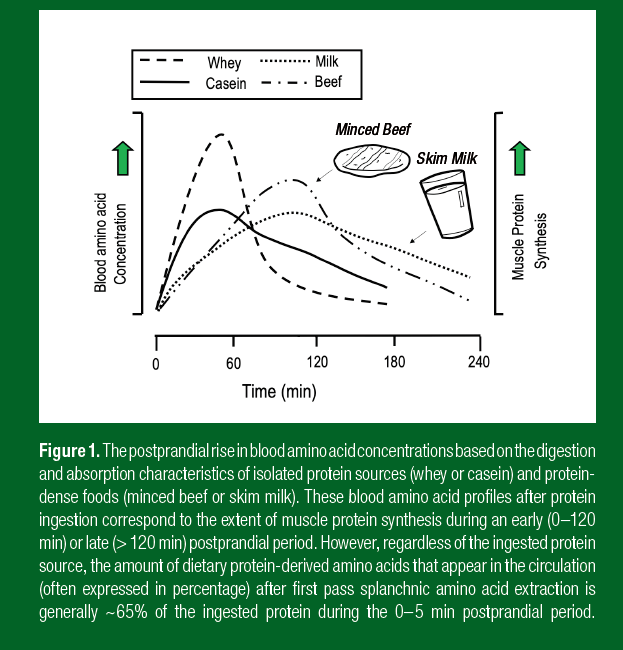 As shown in Figure 1, the type of ingested food can differentially modulate the speed and peak amplitude of dietary amino acids in the circulation and the subsequent postprandial MPS response. For example, the time to peak blood amino acid concentrations after whole-food ingestion, such as milk or minced beef, is delayed when compared to isolated protein sources such as whey or casein. Whey protein ingestion, due to its high leucine content and high solubility, results in the rapid (with high peak amplitude) delivery of dietary amino acids into the circulation during the postprandial period. Consequently, most of the dietary protein-derived amino acids are absorbed during the early postprandial phase and available to stimulate a robust increase in MPS in a relatively short time frame (~3–4 h) (Pennings et al., 2011). For this reason, the ingestion of isolated protein supplements is popular to stimulate the MPS response immediately post-exercise. However, isolated protein supplements co-ingested with carbohydrate attenuates the amplitude of the postprandial rise in plasma amino acid concentrations (Staples et al., 2011). While carbohydrate co-ingestion with protein does not potentiate the use of dietary protein-derived amino acids in circulation for post-exercise MPS (Staples et al., 2011), carbohydrate intake during the recovery period is necessary to promote muscle glycogen resynthesis rates during recovery and, ultimately, optimal performance for both weightlifting and endurance athletes. As shown in Figure 1, whole-food sources of protein, such as milk or meat ingestion, results in a slower rise in peak dietary protein availability and a more prolonged release during the postprandial period (> 5 h) (Burd et al., 2015). Thus, a large part of the day is spent in the postprandial period, especially when eating food combinations including carbohydrate and fat.
As shown in Figure 1, the type of ingested food can differentially modulate the speed and peak amplitude of dietary amino acids in the circulation and the subsequent postprandial MPS response. For example, the time to peak blood amino acid concentrations after whole-food ingestion, such as milk or minced beef, is delayed when compared to isolated protein sources such as whey or casein. Whey protein ingestion, due to its high leucine content and high solubility, results in the rapid (with high peak amplitude) delivery of dietary amino acids into the circulation during the postprandial period. Consequently, most of the dietary protein-derived amino acids are absorbed during the early postprandial phase and available to stimulate a robust increase in MPS in a relatively short time frame (~3–4 h) (Pennings et al., 2011). For this reason, the ingestion of isolated protein supplements is popular to stimulate the MPS response immediately post-exercise. However, isolated protein supplements co-ingested with carbohydrate attenuates the amplitude of the postprandial rise in plasma amino acid concentrations (Staples et al., 2011). While carbohydrate co-ingestion with protein does not potentiate the use of dietary protein-derived amino acids in circulation for post-exercise MPS (Staples et al., 2011), carbohydrate intake during the recovery period is necessary to promote muscle glycogen resynthesis rates during recovery and, ultimately, optimal performance for both weightlifting and endurance athletes. As shown in Figure 1, whole-food sources of protein, such as milk or meat ingestion, results in a slower rise in peak dietary protein availability and a more prolonged release during the postprandial period (> 5 h) (Burd et al., 2015). Thus, a large part of the day is spent in the postprandial period, especially when eating food combinations including carbohydrate and fat.Overall, dietary amino acids are direct stimulators of MPS; thus, it is possible to modulate the elevation of the post-exercise MPS response during recovery by manipulating the peak rise in plasma amino acid availability after protein ingestion. This is particularly relevant when consuming isolated protein sources or even protein-dense foods. The significance of stimulating an early (0–2 h) versus late (2–5 h) post-exercise rise in MPS, as observed with ingestion of isolated protein sources and protein dense foods, respectively, is not clear. However, there is a prolonged window of anabolic opportunity after exercise that persists for 1–2 days (Burd et al., 2011). Thus, training-induced adaptations are facilitated through the consistent and conscious habit of incorporating protein at every meal through this prolonged recovery period.
FOOD MATRIX EFFECTS TO FACILITATE MUSCLE ANABOLIC POTENTIAL
The holistic properties of foods (food synergy) and their influence on post-exercise muscle protein remodeling and repair has received little attention. Dietary protein is often more than just its constituent amino acids, containing other non-protein nutritive components that may interact with nutrients, modulate nutrient behavior, and/or act directly as signaling molecules. In the case of some foods, such as yogurt, they may also contain living organisms or live and active cultures which synergistically enhance the health impact of the food source.
The food matrix describes the overall physical form of food and includes how various food components are structured and may interact. Food processing and heat treatment can have direct effects on the food matrix and modulate protein digestibility (Evenepoel et al., 1998). While reductionist thinking has uncovered the most fundamental anabolic components (i.e., essential amino acids) of food to stimulate post-exercise MPS (Volpi et al., 2003), this may not accurately reflect food matrix effects experienced in a mixed-meal setting of whole foods. Thus, we may be missing an opportunity to optimize protein in the diet for an athlete. In other words, the food matrix in which protein is consumed may have a modulatory effect on post-exercise MPS.
There has been direct evidence to demonstrate that a food matrix rich in dietary proteins, vitamins, minerals, and other macronutrients (e.g., whole milk or eggs) modulates the post-exercise MPS synthetic response when compared to eating more protein-dense foods (Elliot et al., 2006; Burd et al., 2015; van Vliet et al., 2017). Interestingly, attempts have been made to isolate food components and subsequently combine them with isolated protein sources to potentially potentiate the postprandial MPS response. For example, studies combining micellar casein ingestion with individual food components such as milk fat (Gorissen et al., 2017), carbohydrates (Gorissen et al., 2014), or milk serum (mixture of 10% lactose, 0.3% protein, 0.06% fat, and 1.1% minerals) (Churchward-Venne et al., 2015) were unable to further increase the postprandial MPS response when compared to ingestion of micellar casein alone. Hence, there seems to be superior postprandial MPS responses when eating whole foods versus extraction of specific nutrients into a processed product.
However, these observations do not disregard the effectiveness of isolated protein sources, particularly when consumed in sufficiently high quantities on post-exercise MPS (Moore et al., 2009a). However, ingesting a food matrix rich in protein, macro-, and micro-nutrients potentially strengthens the use of dietary protein-derived amino acids for post-exercise MPS (Elliot et al., 2006; Burd et al., 2015; van Vliet et al., 2017). Not only will this ultimately help improve the use of dietary protein for an athlete, but also whole-food ingestion will improve overall diet quality. Moreover, female athletes who are more prone to energy and micronutrient deficiencies (i.e., iron, calcium, vitamin D and B) may benefit from prioritizing the ingestion of protein-rich whole food over protein supplements during recovery from exercise. Of course, food selection is also dependent on the competitive/training schedule, prevalence of GI problems, cost, and food availability among other factors.
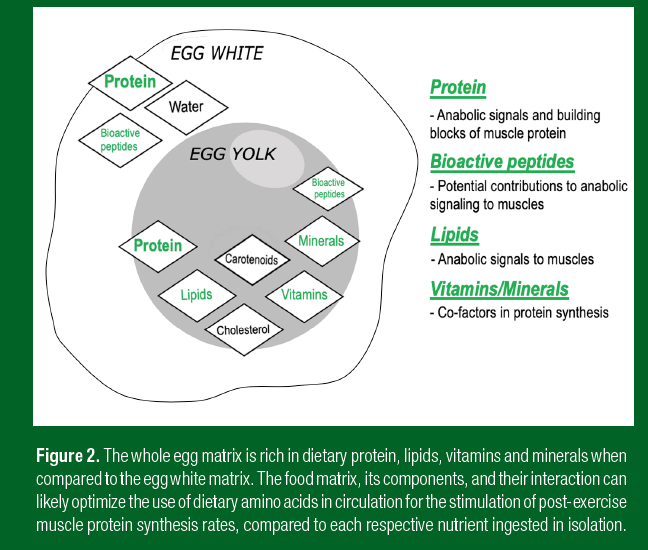
Figure 2 illustrates the difference between a whole egg matrix, which is rich in high-quality dietary protein, lipids, vitamins and minerals, when compared to an egg white matrix. The latter food matrix is fairly protein-dense, but contains very few other nutritive constituents. In addition, Figure 2 lists the potential mechanisms that describe how the individual food constituents may create a synergistic environment to potentiate the use of dietary protein-derived amino acids for the post-exercise MPS response. It is noteworthy that the overall energy content of a protein meal co-ingested with other isolated macronutrients, or its associated postprandial plasma insulin response, has not been shown to be an anabolic factor for MPS in vivo in humans (Gorissen et al., 2014; 2017). Thus, the anabolic properties of foods on MPS extends beyond their energy content and associated rise in plasma insulin concentrations in the face of elevated plasma amino acid availability (Elliot et al., 2006). Future work is required to further define how whole-food sources of protein can be used to optimize protein in a meal for an athlete.
SUMMARY AND PRACTICAL APPLICATIONS
In summary, an acute bout of exercise increases the anabolic potential of post-exercise meals throughout a prolonged recovery period. Protein is the fundamental anabolic component of post-exercise nutrition to promote MPS responses that provide the basis for muscle and performance adaptations. Ensuring adequate intake of high-quality protein during post-exercise recovery is a priority, and utilizing proteinrich whole foods or protein supplements highly depends on both nutritional (amino acid profile or nutrient density) and non-nutritional (i.e., preference, cost, availability) factors, and how a particular feeding paradigm would complement the recovery strategy for different types of training and competitive events.
- The maximal amount of protein to consume in a meal after resistance exercise has been shown to range from 20–40 g of protein based on isolated sources. This only defines maximal stimulation of MPS, and more efficient dosing has yet to be determined.
- Practical applications of maximal protein dosing for MPS based on isolated protein sources (i.e., 20–40 g per meal) may be challenging to achieve when considering other nutrient requirements.
- Defining the optimal protein dosing seeks to maximize the use of dietary amino acids for MPS while minimizing amino acid loss to oxidation.
- The ingestion of whole-food sources of protein, due to the interaction of their non-protein nutritive components, can likely optimize the use of dietary amino acids for post-exercise MPS. In addition, diet quality can be improved through the ingestion of whole-food sources of protein when compared to isolated protein sources.
- Sports dietitians must take into consideration the typical eating pattern and food choice of an athlete (i.e., some populations obtain most of their dietary protein from plant-based whole foods) when creating nutrition recommendations, as this may be useful in identifying whether to incorporate protein-rich whole foods, supplements, or both.
REFERENCES
Abou Sawan, S., S. van Vliet, J.T. Parel, J.W. Beals, M. Mazzulla, D.W.D. West, A. Philp, Z. Li, S.A. Paluska, N.A. Burd, and D.R. Moore (2018). Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol. Rep. 6(5):e13628.
Allen, J.M., L.J. Mailing, G.M. Niemiro, R. Moore, M.D. Cook, B.A. White, H.D. Holscher, and J.A. Woods (2018). Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50:747-757.
An, R. and N.A. Burd (2015). Change in daily energy intake associated with pairwise compositional change in carbohydrate, fat and protein intake among US adults, 1999-2010. Pub. Health Nutr 18:1343-1352.
Biolo, G., K.D. Tipton, S. Klein, and R.R. Wolfe (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 273:E122-E129.
Burd, N.A., S.H. Gorissen, S. van Vliet, T. Snijders, and L.J. van Loon (2015). Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am. J. Clin. Nutr 102:828-836.
Burd, N.A., D.W. West, D.R. Moore, P.J. Atherton, A.W. Staples, T. Prior, J.E. Tang, M.J. Rennie, S.K. Baker, and S.M. Phillips (2011). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J. Nutr. 141, 568-573.
Churchward-Venne, T.A., T. Snijders, A.M. Linkens, H.M. Hamer, J. van Kranenburg, and L.J. van Loon (2015). Ingestion of casein in a milk matrix modulates dietary protein digestion and absorption kinetics but does not modulate postprandial muscle protein synthesis in older men. J. Nutr. 145:1438-1445.
Elliot, T.A., M.G. Cree, A.P. Sanford, R.R. Wolfe, and K.D. Tipton (2006). Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med. Sci. Sports Exerc. 38:667-674.
Evenepoel, P., B. Geypens, A. Luypaerts, M. Hiele, Y. Ghoos, and P. Rutgeerts (1998). Digestibility of cooked and raw egg protein in humans as assessed by stable isotope techniques. J. Nutr. 128:1716-1722.
Gorissen, S.H., N.A. Burd, H.M. Hamer, A.P. Gijsen, B.B. Groen, and L.J. van Loon (2014). Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin Endocrinol. Metab. 99:2250-2258.
Gorissen, S.H.M., N.A. Burd, I.F. Kramer, J. van Kranenburg, A.P. Gijsen, O. Rooyackers, and L.J.C. van Loon (2017). Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin. Nutr. 36:429-437.
Jacobs, D.R., Jr., M.D. Gross, and L.C. Tapsell (2009). Food synergy: an operational concept for understanding nutrition. Am. J. Clin. Nutr. 89:1543S-1548S.
JanssenDuijghuijsen, L.M., M. Mensink, K. Lenaerts, E. Fiedorowicz, Protégé study group, D.A. van Dartel, J.J. Mes, Y.C. Luiking, J. Keijer, H.J. Wichers, R.F. Witkamp, and K. van Norren (2016). The effect of endurance exercise on intestinal integrity in well-trained healthy men. Physiol. Rep 4(20):e12994.
Mazzulla, M., J.T. Parel, J.W. Beals, S. van Vliet, S. Abou Sawan, D.W.D. West, S.A. Paluska, A.V. Ulanov, D.R. Moore, and N.A. Burd (2017). Endurance exercise attenuates postprandial whole-body leucine balance in trained men. Med. Sci. Sports Exerc. 49:2585-2592.
Meyer, N. and A. Reguant-Closa (2017). “Eat as if you could save the planet and win!” Sustainability integration into nutrition for exercise and sport. Nutrients 9:412.
Moore, D.R., M.J. Robinson, J.L. Fry, J.E. Tang, E.I. Glover, S.B. Wilkinson, T. Prior, M.A. Tarnopolsky, and S.M. Phillips (2009a). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89:161-168.
Moore, D.R., J.E. Tang, N.A. Burd, T. Rerecich, N.A. Tarnopolsky, and S.M. Phillips (2009b). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J. Physiol. 587:897-904.
Pennings, B., Y. Boirie, J.M. Senden, A.P. Gijsen, H. Kuipers, and L.J. van Loon (2011). Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 93:997-1005.
Raj, T., U. Dileep, M. Vaz, M.F. Fuller, and A.V. Kurpad (2008). Intestinal microbial contribution to metabolic leucine input in adult men. J. Nutr. 138:2217-2221.
Staples, A.W., N.A. Burd, D.W. West, K.D. Currie, P.J. Atherton, D.R. Moore, M.J. Rennie, M.J. Macdonald, S.K. Baker, and S.M. Phillips (2011). Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med. Sci. Sports Exerc. 43:1154-1161.
Tang, J.E., D.R. Moore, G.W. Kujbida, M.A. Tarnopolsky, and S.M. Phillips (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 107:987-992.
van Vliet, S., N.A. Burd, and L.J. van Loon (2015). The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 145:1981-1991.
van Vliet, S., E.L. Shy, S. Abou Sawan, J.W. Beals, D.W. West, S.K. Skinner, A.V. Ulanov, Z. Li, S.A. Paluska, C.M. Parsons, D.R. Moore, and N.A. Burd (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr. 106:1401-1412.
van Wijck, K., B. Pennings, A.A. van Bijnen, J.M. Senden, W.A. Buurman, C.H. Dejong, L.J. van Loon, and K. Lenaerts (2013). Dietary protein digestion and absorption are impaired during acute postexercise recovery in young men. Am. J. Physiol. 304:R356-R361.
Volpi, E., H. Kobayashi, M. Sheffield-Moore, B. Mittendorfer, and R.R. Wolfe (2003). Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 78:250-258.








