Promoting Optimal Omega-3 Fatty Acid Status in Athletes
Published
March 2021
Author
Peter Ritz MS, RD, CSSD; Michelle Rockwell Ph.D., RD, CSSD
Topics
Training & Performance , Supplements , Recovery , Protein , Sports Nutrition , Athlete Health
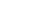
KEY POINTS
- Omega-3 fatty acids (O3FA) have been shown to impact the health and performance of athletes in numerous ways, including the management of inflammation, enhancement of muscle recovery, and protection of brain health and function.
- Dietary recommendations for O3FA are highly variable, which creates a particular challenge in determining athlete-specific needs. Low O3FA status has been observed among multiple athletic populations.
- Dietary sources of O3FA include eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and alpha-linolenic acid (ALA). Sources of EPA and DHA are limited in the food supply, with fatty fish and seafood being the predominant sources. Dietary sources of ALA are more common, but endogenous conversion of ALA to EPA and DHA is generally believed to be modest at best, emphasizing the value of incorporating fish and seafood into an athlete’s diet.
- EPA and DHA supplementation may be required for athletes to achieve optimal O3FA status. An intake of 1–3 g of EPA + DHA daily, including both dietary sources and supplements, is a reasonable goal that could provide benefits to athletes with low risk of undesirable side effects. When selecting an O3FA supplement, the O3FA source, form, dose, as well as a variety of athlete-specific factors should be considered.
- Further research is needed to better understand the role of O3FA in the health and performance of athletes, and to identify athlete-specific O3FA recommendations.
INTRODUCTION
Omega-3 fatty acids (O3FA) are a group of unsaturated fats characterized by a third-carbon double bond within their biochemical structure. Although there are several different O3FA, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and alpha-linolenic acid (ALA) are most prominent and most thoroughly researched in terms of human physiology and metabolism. The majority of health and performance benefits related to consumption of O3FA have been associated with EPA and DHA. While it is possible for ALA (found primarily in plant-based sources) to be converted to EPA and DHA in the body, the rate of conversion is modest, at best (Arterburn et al., 2006; Metherel & Bazinet, 2019). Thus, direct consumption of EPA and DHA (found primarily in marine-based sources) is the best strategy for attaining these nutrients.
Based on dietary analysis (Ritz et al., 2020; Wilson & Madrigal, 2016) and assessment of blood O3FA concentrations (Anzalone et al., 2019; Davinelli et al., 2019; Ritz et al., 2020), a high proportion of athletes appear to have low O3FA status. Opportunities to improve athletes’ O3FA status through dietary sources and supplements exist. One barrier to supplementation in sport was overturned in 2019 when O3FA supplements were reclassified by the National Collegiate Athletic Association (NCAA) as permissible for Division I athletic departments to provide to student-athletes. Nevertheless, making decisions related to O3FA for athletes is complicated by the lack of athlete-specific O3FA guidelines, numerous factors related to dietary sources and supplements, inconsistencies in the literature, and evolving research. The purpose of this Sports Science Exchange article is to discuss practical issues related to the promotion of optimal O3FA status in athletes.
OMEGA-3 FATTY ACIDS IN THE HEALTH AND PERFORMANCE OF ATHLETES
As a component of cell membrane phospholipids, O3FA may influence the composition and function of many tissues throughout the body, including cardiovascular, brain, skeletal muscle and immune tissue (Witard and Davis (2021) SSE#211; Gerling et al., 2019; Shahidi & Ambigaipalan, 2018). O3FA are also known to mitigate inflammation (Heaton et al., 2017). Associations between O3FA status and risk of cardiovascular disease, type 2 diabetes, cancer, arthritis and cognitive decline exist, although not all studies show benefits of supplementation for individuals with these conditions (Nichols et al., 2014; Shahidi & Ambigaipalan, 2018).
There is also evidence linking O3FA status to health and performance benefits for athletes. A recent systematic review identified 32 studies related to O3FA supplementation and various markers of physiology and performance in athletes (Lewis et al., 2020). Overall, a positive association between O3FA supplementation and reaction time, skeletal muscle recovery, inflammatory markers and cardiovascular dynamics was reported (Lewis et al., 2020). Supplementation has also been shown to influence muscle protein synthesis, especially in conditions such as immobilization and energy restriction, or when consumed with other nutrients (Black et al., 2018; McGlory et al., 2016). Finally, a role for O3FA (particularly DHA) in the prevention and treatment of traumatic brain injury/concussion has been identified, and continues to be investigated (Barrett et al., 2014; Oliver et al., 2016).
One mechanism by which O3FA can impact health and performance relates to the balance between omega-6 fatty acids (O6FA) and O3FA in the body. Although both are essential nutrients, a high O6FA:O3FA ratio has been associated with increased inflammation, thrombosis and dysregulation of metabolic health (McGlory et al., 2019). Sources of O6FA include plant oils such as soybean and corn, many highly processed foods (e.g., salad dressings, margarines, snack foods), some nuts and seeds, and grain-fed meat and dairy products. Modern diets, particularly in North America, have evolved to contain substantially more O6FA than O3FA. An average O6FA:O3FA ratio of ~15:1 has been reported in the American diet, whereas a ratio of 4:1 or less is often recommended (Simopoulos, 2002). Worth noting, the arachidonic acid (AA):EPA ratio has been suggested as a potentially more relevant indicator of balance between O6FA and O3FA in the diet since AA and EPA compete metabolically for eicosanoid production (Davinelli et al., 2020).
The reader is referred to a complementary Sports Science Exchange (SSE) article by Witard and Davis (2021), Oliver et al., (2018), Philpott et al., (2019) and Mickleborough (2013), for further reading on the role of O3FA in the health and performance of athletes.
DAILY REQUIREMENTS FOR OMEGA-3 FATTY ACIDS: MINIMUM, OPTIMAL AND MAXIMUM
Interestingly, no Recommended Dietary Allowance (RDA) or Daily Value (DV) guidelines for O3FA have been established. However, several dietary recommendations exist. The Academy of Nutrition and Dietetics and Dietitians of Canada, for example, recommend consumption of 0.5 g EPA + DHA daily, whereas the European Food Safety Authority recommends 0.25 g EPA + DHA daily (European Food Safety Authority, 2012; Omega-3 Global Intake Recommendations by Country, 2014; Vannice & Rasmussen, 2014). The American Heart Association recommends that healthy individuals meet O3FA needs through eating two > 3.5-ounce servings of fish weekly, but that individuals with coronary heart disease target 1 g EPA + DHA daily and those with elevated serum triglycerides aim for 2-4 g EPA + DHA each day (Siscovick et al., 2017).
Of note, none of the above recommendations are athlete-specific and many are based on a potential relationship between O3FA and coronary heart disease. Athletes likely require more O3FA than the general population with factors such as sex, body weight, energy metabolism, training volume and the inflammatory response to exercise all influencing needs (Davinelli et al., 2019; Drobnic et al., 2017; Flock et al., 2013; Tepsic et al., 2009; Walker et al., 2019b). Furthermore, the minimum effective level of O3FA for health and performance may differ from the optimal level. Therapeutic and ergogenic benefits have commonly been associated with higher doses obtained through supplements, since achieving high levels from diet alone is difficult.
Recommendations concerning the maximum amount of O3FA appropriate for daily consumption are also variable. The U.S. National Academy of Medicine and European Food Safety Authority have not established an upper intake level for O3FA (Global Organization for EPA & DHA, 2014). As O3FA are known to play a role in thrombosis, concern about increased bleeding risk with O3FA supplementation has been reported. However, a recent systematic review did not identify surgery-related bleeding risk in healthy individuals taking O3FA supplements (Begtrup et al., 2017). Other potential consequences of excessive O3FA intake include elevated low density lipoprotein (LDL)-cholesterol and various gastrointestinal symptoms (Bradberry & Hilleman, 2013). Overall, up to 5 g of EPA+DHA daily has been described as generally well-tolerated and not associated with adverse complications (European Food Safety Authority, 2012).
Do Athletes Consume Optimal Levels of O3FA?
Athletes’ diets have been shown to contain sub-optimal levels of O3FA. Based on dietary assessment (targeted food-frequency questionnaire) of more than 1,500 athletes from nine NCAA Division I athletic programs, Ritz et al., (2020) observed that less than 40% of athletes met recommendations to consume fish or seafood at least twice weekly and less than 10% of athletes met the Academy of Nutrition and Dietetics’ recommendation to consume >0.5 g EPA + DHA daily. Wilson and Madrigal (2016) reported similar findings in collegiate athletes.
O3FA status can also be determined via assessment of blood biomarkers. In general, plasma and serum fatty acids are uncommonly used to evaluate O3FA status since concentrations are influenced by recent dietary consumption. Omega-3 Index (O3i) has been increasingly used in research, clinical and practical settings as a biomarker of long-term O3FA status. The O3i reflects the EPA + DHA content of red blood cell (RBC) membranes, expressed as a percentage of total RBC fatty acids. Benefits of this biomarker are that it corresponds with dietary intake and tissue content, requires a minimal amount of blood (i.e., finger stick blood spot sample), and has low biological variability (Harris & Thomas, 2010). An O3i of > 8% is associated with the lowest risk of cardiovascular disease (Harris, 2007). There is also some evidence supporting an association between O3i and cognitive function in non-athletes (Cook et al., 2019). Multiple studies have identified an average O3i of 3-4% in athletes (Anzalone et al., 2019; Davinelli et al., 2019; Ritz et al., 2020; von Schacky et al., 2014; Wilson & Madrigal, 2016). Assessment of O3i may be valuable in screening for sub-optimal O3FA status, designing individualized treatment protocols, and evaluating responses to treatment.
DIETARY SOURCES OF OMEGA-3 FATTY ACIDS
EPA and DHA
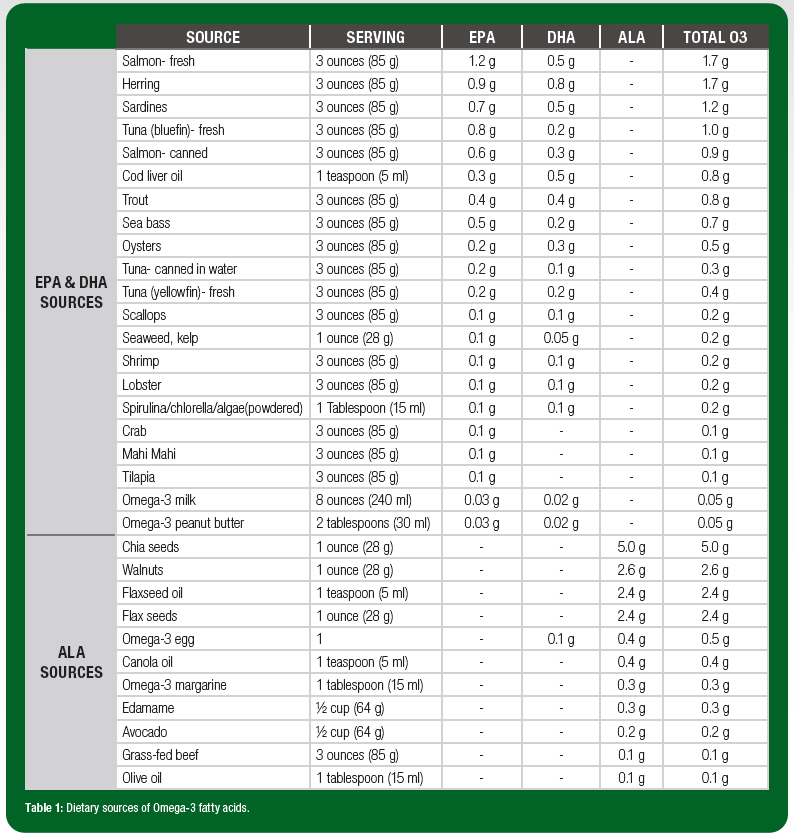
Algae, phytoplankton and other marine microorganisms are natural producers of EPA and DHA. In turn, fish and seafood that consume these microorganisms are the richest sources in the food supply. However, there is substantial variation in O3FA content of these food sources (Table 1). For example, fatty fish such as salmon, sardines and bluefin tuna have at least 1 g of EPA + DHA per 3-oz serving. Popular sources such as shrimp, scallops and canned tuna, on the other hand, contain far less (<0.2 g EPA + DHA in a 3-oz serving). Table 2 illustrates portion sizes of common foods that provide 0.5-1 g EPA + DHA.
ALA
Food sources of ALA include walnuts, chia and flax, and vegetable/seed oils (Table 1). Grass-fed meats and eggs also contain ALA as a byproduct of animals’ diets. Although these food sources are often recommended as a means of enhancing dietary O3FA, they do not contain EPA or DHA (the O3FA most associated with health and performance benefits) and the capacity to convert ALA to EPA and DHA is relatively low in human physiology (Arterburn et al., 2006; Plourde & Cunnane, 2007), although some recent evidence suggests conversion rates may be higher than previously estimated (Metherel & Bazinet, 2019). It is also possible that individuals who do not consume fish or seafood experience a greater ALA conversion, though this is not conclusive in the literature. Athletes who follow a vegetarian diet, have fish or seafood allergies, or prefer not to consume fish or seafood may benefit from consumption of seaweed, kelp, algae, fortified foods, or from O3FA supplementation (algae-based).
OMEGA-3 SUPPLEMENTATION
Dietary supplementation is another approach to improving O3FA status. In addition to the recommendation to use third-party tested products for purity and safety purposes, several additional factors may be considered.
Product Types
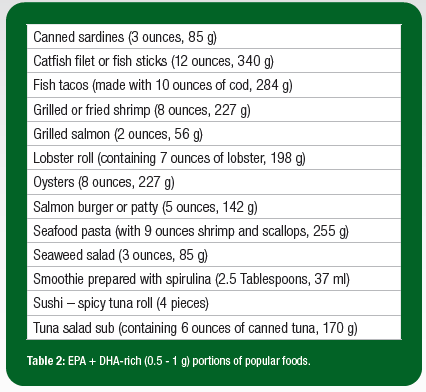
O3FA supplements come from a variety of sources (fish, krill and algae being the most common) and contain a variety of lipid forms (ethyl ester, triglyceride, phospholipid and free fatty acid) that practitioners should be familiar with when evaluating products (Table 3). Crude fish oil originates directly from the tissue of oily fish and contains less than 30% O3FA. The majority of available products are non-concentrates, while others undergo more rigorous processing designated as O3FA concentrates. Fish oil supplements most often include O3FA in the ethyl ester form, and sometimes, in the triglyceride, phospholipid and free fatty acid forms. Krill oil supplements, which contain oil extracted from Antarctic krill, have become increasingly popular due to their higher concentrations of phospholipid and free fatty acid forms. Krill oil also contains an antioxidant called astaxanthin, which prevents the oxidation of O3FA and is associated with optimal structure and function of the eyes (Barros et al., 2014). Algae oil is a plant-based alternative, in the triglyceride form and may particularly appeal to vegetarian athletes.
Bioavailability and Tissue Incorporation
Different lipid forms (ethyl ester, free fatty acid, phospholipid and triglyceride) vary in terms of bioavailability and incorporation into target tissues (Table 3). Several studies suggest that the bioavailability of ethyl ester is inferior to other forms, observed to be less effective at both increasing O3i and reducing triglyceride levels (Ghasemifard et al., 2014; Neubronner et al., 2011; Schuchardt et al., 2011). Using animal models, there is some evidence that a phospholipid form may be preferentially incorporated into tissues like the eyes and brain (Liu et al., 2014), but there is insufficient data available to make conclusions in humans. Based on the available evidence, a triglyceride-based supplement derived from fish oil or algae oil may be the best recommendation for many athletes at this time, as those in the free fatty acid form are highly susceptible to oxidation and phospholipid products come in comparatively smaller dosages, increasing the cost per serving (Schuchardt & Hahn, 2013). Consumers should also be instructed to take supplements with food, as improved absorption has been observed when supplements are taken with a fat-containing meal (Lawson & Hughes, 1988).
Active Ingredient vs. Total Ingredients
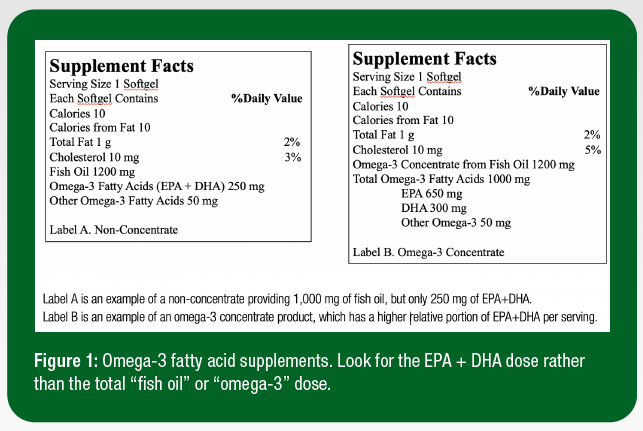
When evaluating the cost efficiency of products, it is imperative to consider the product’s EPA + DHA content rather than “fish oil” or “O3FA” content and differentiate between a concentrate and a non-concentrate supplement (Figure 1). An omega-3 concentrate typically provides a larger dose of EPA and DHA per serving to provide a more cost-effective intervention.
Determining Dosage Specific to the Individual
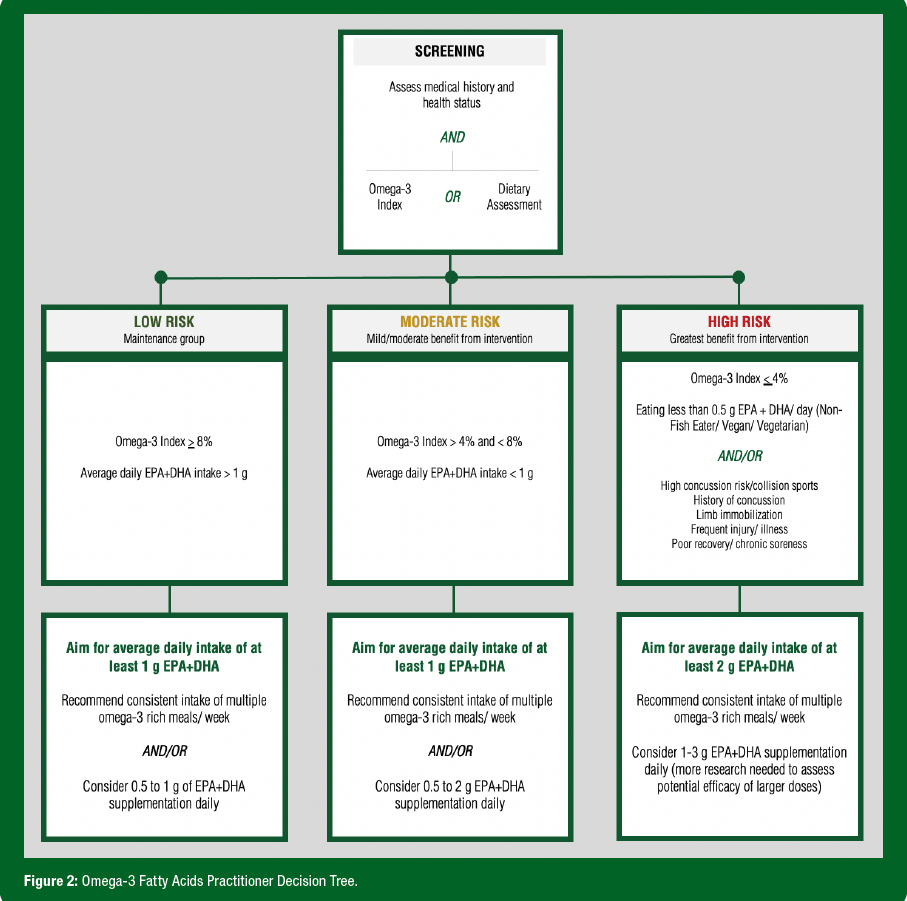
There are many factors and approaches to consider in determining an O3FA supplement dose specific to the athlete. Factors such as habitual diet, sex, age, body weight, training load, smoking status and others may all influence recommendations. Figure 2 outlines an approach to dosing based on screening and classification of risk.
One approach for supplementation is to aim for an O3i of 8% or greater. Assuming an O3i baseline near the American average of 4-5%, a 1 g/day dose of EPA + DHA for 20 weeks (Flock et al., 2013) or a 2 g/day dose of EPA + DHA for 13 weeks (Walker et al., 2019a) has been observed to achieve an O3i of >8% in healthy adult subjects. While more research is needed to assess athlete-specific dose response, a preliminary study observed that Olympic athletes required 1.5-2 g/day of EPA + DHA for at least 16 weeks to accomplish the O3i benchmark of >8% (Drobnic et al., 2017). Once the goal O3i has been achieved, a smaller maintenance dose may be considered.
Another approach is to select a dose specific to the goal of supplementation. For example, 1 g EPA + 2 g DHA daily is a common recommendation for athletes when neuroprotection is the primary goal, given the near maximal plasma response seen observed with a 2 g/day DHA dose (Arterburn et al., 2006; Oliver et al., 2018). Body weight-based dosing, using up to 40 mg/kg as a reference, has also been recommended, based on effective doses in animal research (Flock et al., 2013; Mills et al., 2011). Overall, it is important to note that significant variation in individual dose response exists (Walker et al., 2019a).
SUMMARY AND ACTION STEPS
- Although O3FA are known to influence athletes’ health and performance, much remains to be learned. Practitioners should commit to staying up to date with research and athlete-specific recommendations, and ideally, participating in applied research.
- Consistent athlete-specific recommendations for dietary or supplemental O3FA are not available. The minimal intake required to support health of the general population may differ substantially from the optimal intake required for athletes seeking to enhance health and performance.
- As most athletes have been shown to consume sub-optimal O3FA, numerous opportunities to improve athletes’ O3FA status exist. Incorporating menu planning strategies to promote frequent consumption of EPA + DHA-rich foods while negotiating budget limitations, individual preferences and food availability is an important role for the nutrition practitioner
- Achieving optimal O3FA status may require supplementation. Available O3FA supplements vary in source, form and dosage. Given the available evidence to date, a concentrated triglyceride-based fish oil or algae oil supplement may be the best option for many athletes.
- Since numerous factors influence O3FA status and response to supplementation, individualized supplement dosing is recommended whenever possible. Figure 2 highlights one approach to individualizing supplement recommendations.
- In the situation when a standardized protocol is more practical than individualized recommendations, a daily dose of 1-3 g EPA + DHA may be appropriate. Some may consider a higher dose for higher body weight athletes, during intense training periods, or when neuroprotection is the primary goal.
- Assessing O3FA status via measurement of the RBC omega-3 index (O3i) may be useful in screening athletes, tailoring supplement recommendations and evaluating dose response. If O3i measurement is not possible, practitioners may consider using validated dietary assessment tools to evaluate typical O3FA intake.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Anzalone, A., A. Carbuhn, L. Jones, A. Gallop, A. Smith, P. Johnson, L. Swearingen, C. Moore, E. Rimer, J. McBeth, W. Harris, K.M. Kirk, D. Gable, A. Askow, W. Jennings, and J.M. Oliver (2019). The Omega-3 Index in National Collegiate Athletic Association division I collegiate football athletes. J. Athl. Train. 54:7–11.
Arterburn, L.M., E.B. Hall, and H. Oken (2006). Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83(6 Suppl):1467S-1476S.
Barrett, E.C., M.I. McBurney, and E.D. Ciappio (2014). ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv. Nutr. 5:268–277.
Barros, M.P., S.C. Poppe, and E.F. Bondan (2014). Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 6:1293–1317.
Begtrup, K.M., A.E. Krag, and A.-M. Hvas (2017). No impact of fish oil supplements on bleeding risk: A systematic review. Danish Med. J. 64:5.
Black, K.E., O.C. Witard, D. Baker, P. Healey, V. Lewis, F. Tavares, S. Christensen, T. Pease, and B. Smith (2018). Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport Sc. 18:1357–1367.
Bradberry, J.C., and D.E. Hilleman (2013). Overview of omega-3 fatty acid therapies. Pharmacy and therapeutics. 38:681–691.
Cook, R.L., H.M. Parker, C.E. Donges, N.J. O’Dwyer, H.L. Cheng, K.S. Steinbeck, E.P. Cox, J.L. Franklin, M.L. Garg, and H.T. O’Connor (2019). Omega-3 polyunsaturated fatty acids status and cognitive function in young women. Lipids Health Dis. 18:194.
Davinelli, S., G. Corbi, S. Righetti, E. Casiraghi, F. Chiappero, S. Martegani, R. Pina, L. De Vivo, A.P. Simopoulos, and G. Scapagnini (2019). Relationship between distance run per week, omega-3 index, and arachidonic acid (AA)/eicosapentaenoic acid (EPA) ratio: An observational retrospective study in non-elite runners. Front. Physiol. 10:487.
Davinelli, S., M. Intrieri, G. Corbi, and G. Scapagnini (2020). Metabolic indices of polyunsaturated fatty acids: Current evidence, research controversies, and clinical utility. Crit. Rev. Food Sci. Nutr. 1–16.
Drobnic, F., F. Rueda, V. Pons, M. Banquells, B. Cordobilla, and J.C. Domingo (2017). Erythrocyte omega-3 fatty acid content in elite athletes in response to omega-3 supplementation: A dose-response pilot study. J. Lipids. 1472719.
European Food Safety Authority (2012). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 10:2815.
Flock, M.R., A.C. Skulas‐Ray, W.S. Harris, T.D. Etherton, J.A. Fleming, and P.M. Kris‐Etherton (2013). Determinants of erythrocyte omega‐3 fatty acid content in response to fish oil supplementation: A dose–response randomized controlled trial. J. Am. Heart Assoc: Cardiovascular and Cerebrovascular Disease. 2:6.
Gerling, C.J., K. Mukai, A. Chabowski, G.J.F. Heigenhauser, G.P. Holloway, L.L. Spriet, and S. Jannas-Vela (2019). Incorporation of omega-3 fatty acids into human skeletal muscle sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front. Physiol. 10:348.
Ghasemifard, S., G.M. Turchini, and A.J. Sinclair (2014). Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Progr. Lipid Res. 56:92–108.
Global Organization for EPA and DHA (2014). Omega-3 global intake recommendations by country. https://goedomega3.com/intake-recommendations.
Harris, W.S. (2007). Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 55:217–223.
Harris, W.S., and R.M. Thomas (2010). Biological variability of blood omega-3 biomarkers. Clin. Biochem. 43:338–340.
Heaton, L.E., J.K. Davis, E.S. Rawson, R.P. Nuccio, O.C. Witard, K.W. Stein, K. Baar, J.M. Carter, and L.B. Baker (2017). Selected in-season nutritional strategies to enhance recovery for team sport athletes: A practical overview. Sports Med. 47:2201–2218.
Lawson, L.D., and B.G. Hughes (1988). Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem. Biophys, Res. Commun. 156:960–963.
Lewis, N.A., D. Daniels, P.C. Calder, L.M. Castell, and C.R. Pedlar (2020). Are there benefits from the use of fish oil supplements in athletes? A systematic review. Adv. Nutr. 11:1300-1314.
Liu, L., N. Bartke, H. Van Daele, P. Lawrence, X. Qin, H.G. Park, K. Kothapalli, A. Windust, J. Bindels, Z. Wang, and J.T. Brenna (2014). Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J. Lipid Res. 55:531–539.
McGlory, C., S.L. Wardle, L.S. Macnaughton, O.C. Witard, F. Scott, J. Dick, J.G. Bell, S.M. Phillips, S.D.R. Galloway, D.L. Hamilton, and K.D. Tipton (2016). Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 4:6.
McGlory, C., P.C. Calder, and E.A. Nunes (2019). The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 6:144.
Metherel, A.H., and R.P. Bazinet (2019). Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Progr. Lipid Res. 76:101008.
Mickleborough, T.D. (2013). Omega-3 polyunsaturated fatty acids in physical performance optimization. Int. J. Sport Nutr Exerc. Metab. 23:83–96.
Mills, J.D., K. Hadley, and J.E. Bailes (2011). Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 68:474–481.
Neubronner, J., J.P. Schuchardt, G. Kressel, M. Merkel, C. von Schacky, and A. Hahn (2011). Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 65:247–254.
Nichols, P.D., A. McManus, K. Krail, A.J. Sinclair, and M. Miller (2014). Recent advances in omega-3: health benefits, sources, products and bioavailability. Nutrients 6:3727–3733.
Oliver, J.M., K.M. Kirk, D.A. Gable, J.T. Repshas, T.A. Johnson, U. Andreasson, N. Norgren, K. Blennow, and H. Zetterberg (2016). Effect of docosahexaenoic acid on a biomarker of head trauma in american football. Med. Sci. Sports Exerc. 48:974–982.
Oliver, J.M., A.J. Anzalone, and S.M. Turner (2018). Protection before impact: The potential neuroprotective role of nutritional supplementation in sports-related head trauma. Sports Med. 48:39–52.
Philpott, J.D., O.C. Witard, and S.D.R. Galloway (2019). Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 27:219–237.
Plourde, M., and S.C. Cunnane (2007). Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 32:619–634.
Ritz, P.P., M.B. Rogers, J.S. Zabinsky, V.E. Hedrick, J.A. Rockwell, E.G. Rimer, S.B. Kostelnik, M.W. Hulver, and M.S. Rockwell (2020). Dietary and biological assessment of the omega-3 status of collegiate athletes: a cross-sectional analysis. PLoS One. 15:e0228834.
Schuchardt, J.P., J. Neubronner, G. Kressel, M. Merkel, C. von Schacky, and A. Hahn (2011). Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: Results from a six month randomized controlled trial. Prostaglandins Leukotrienes and Essential Fatty Acids. 85:381–386.
Schuchardt, J.P., and A. Hahn (2013). Bioavailability of long-chain omega-3 fatty acids. Prostaglandins, Leukotrienes and Essential Fatty Acids. 89:1–8.
Shahidi, F., and P. Ambigaipalan (2018). Omega-3 polyunsaturated fatty acids and their health benefits. Ann. Rev. Food Sci. Techn. 9:345–381.
Simopoulos, A.P. (2002). The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56:365–379.
Siscovick D.S., T.A. Barringer, A.M. Fretts, J.H.Y. Wu, A.H. Lichtenstein, R.B. Costello, P.M. Kris-Etherton, T.A. Jacobson, M.B. Engler, H.M. Alger, L.J. Appel, and D. Mozaffarian (2017). Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation 135:e867–e884.
Tepsic, J., V. Vucic, A. Arsic, V. Blazencic-Mladenovic, S. Mazic, and M. Glibetic (2009). Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur. J. Appl. Physiol. 107:359–365.
Vannice, G., and H. Rasmussen (2014). Position of the Academy of Nutrition and Dietetics: Dietary fatty acids for healthy adults. journal of the academy of nutrition and dietetics. 114:136–153.
von Schacky, C., M. Kemper, R. Haslbauer, and M. Halle (2014). Low Omega-3 Index in 106 German elite winter endurance athletes: A pilot study. Int. J. Sport Nutr. Exerc. Metab. 24:559–564.
Walker, A.J., B.A. McFadden, D.J. Sanders, M.M. Rabideau, M.L. Hofacker, and S.M. Arent (2019a). Biomarker response to a competitive season in division i female soccer players. J. Strength Cond. Res. 33:2622-2628.
Walker, R.E., K.H. Jackson, N.L. Tintle, G.C. Shearer, A. Bernasconi, S. Masson, R. Latini, B. Heydari, R.Y. Kwong, M. Flock, P.M. Kris-Etherton, A. Hedengran, R.M. Carney, A. Skulas-Ray, S.S. Gidding, A. Dewell, C.D. Gardner, S.M. Grenon, B. Sarter, J.W. Newman, T.L. Pederson, M.K. Larson, and W.S. Harris. (2019b). Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 110:1034-1040.
Wilson, P.B., and L.A. Madrigal (2016). Associations between whole blood and dietary omega-3 polyunsaturated fatty acid levels in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 26:497–505.
Witard, O.C., and Davis J.K. (2021). Omega-3 fatty acids for training adaptation and exercise recovery: a muscle-centric perspective in athletes. SSE #211








