Hydration for Health and Wellness
Published
March 2022
Author
Lindsay B. Baker, PhD; Colin D. Rehm, PhD, MPH; and Michelle A. King, PhD
Topics

KEY POINTS
- Acute decrements in body water (e.g., > 1-2% body mass deficit) can have a detrimental effect on physical and cognitive performance and mood, but effects of habitual fluid intake on general health and wellness outcomes are not well understood.
- Despite wide variation in habitual fluid intake, chronic hypohydration is rare because of homeostatic mechanisms to maintain plasma osmolality. Even within the normal hydration range (< 1% body mass deficit), low-volume drinkers (< 1-2 L/d) may be in a chronic state of renal water reabsorption (underhydration) stimulated by high circulating arginine vasopressin concentrations.
- Emerging research suggests that underhydration may be associated with a higher risk for certain acute conditions and chronic diseases.
- There are relatively robust data from randomized controlled trials suggesting that increasing habitual fluid intake can decrease the risk for recurrent kidney stones and urinary tract infections.
- Fluid restriction may be a risk factor for headache and functional constipation, but there is limited evidence that increasing fluid intake in low-volume drinkers can prevent or treat these conditions.
- For other health outcomes, including chronic kidney disease, metabolic syndrome, and cardiovascular disease, observational epidemiologic studies make up the bulk of the extant research. This makes it difficult to draw direct causal links between fluid intake and disease risk due to concerns about confounding factors and measuring hydration status.
INTRODUCTION
Over the past several decades there has been significant scientific interest in understanding acute deficits in body water through exercise-induced sweating and the associated effects on physiology and performance. Yet, the effects of daily fluid intake habits on general wellness and health outcomes are not well understood. This is because, aside from illness or vigorous exercise, overt hypohydration is usually prevented by homeostatic mechanisms to maintain plasma osmolality and fluid balance via renal water conservation. However, emerging hypotheses suggest that suboptimal habitual fluid intake may be associated with a higher risk for certain acute conditions and chronic diseases (Kavouras, 2019; Perrier, 2017). The purpose of this Sports Science Exchange article is to provide a brief review of the literature and summarize the limitations and future research investigating hydration for health and wellness.
HYDRATION TERMINOLOGY AND REGULATORY PROCESSES
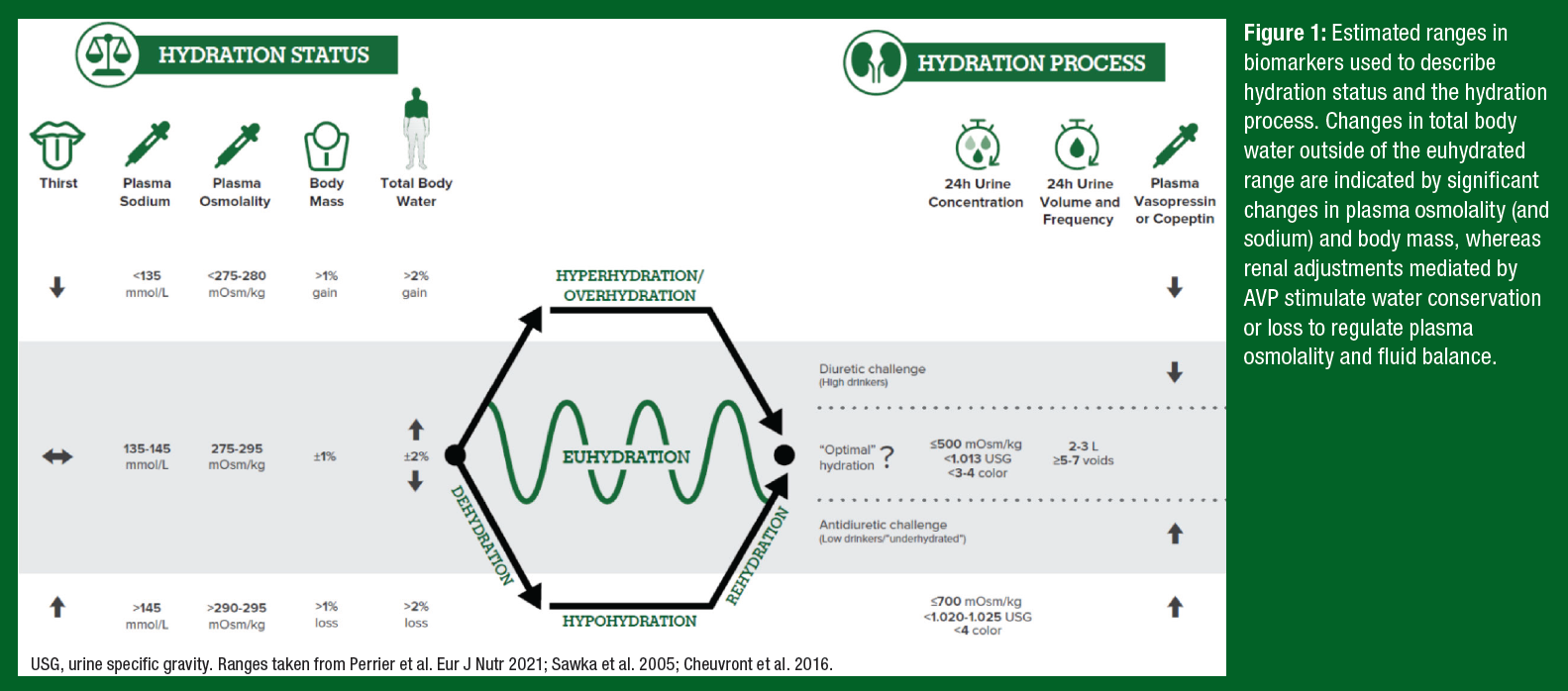
A summary of the terminology around hydration status and the hydration process is illustrated in Figure 1. When there is a significant mismatch between fluid intake and fluid loss (> ±2% total body water (TBW) or ±1% body mass), this imbalance leads to a change in hydration status – i.e., body water deficit (hypohydration) or surplus (hyperhydration). However, in our normal daily lives maintenance of TBW (euhydration) is usually accomplished through tight regulation of plasma osmolality, which is the concentration of dissolved solutes (mostly sodium) in the extracellular fluid compartment. Increases in plasma osmolality stimulate secretion of arginine vasopressin (AVP), resulting in renal water reabsorption (antidiuretic challenge), marked by decreased urine volume and void frequency and increased urine concentration. Thirst is also stimulated with increases in AVP, albeit at a higher osmotic threshold than that of renal water conserving mechanisms (Robertson, 1984).
For reasons just discussed, the hydration process is best assessed through urine volume and concentration. Plasma AVP (or its surrogate, copeptin) may also reflect changes in the hydration process, but threshold values are difficult to establish because of significant biological variability and the need to respond to non-osmotic (nausea, posture, pain, temperature, and circadian factors) as well as osmotic stimuli. While there is no universally accepted gold standard for assessing hydration status, the best indicators of overt hypohydration and overhydration are plasma osmolality and body mass (Cheuvront & Kenefick, 2016). Accordingly, elevated urinary biomarkers are not necessarily indicative of hypohydration. Instead, the term underhydration has been proposed to describe the phenomenon in low-volume drinkers in which water homeostatic mechanisms have been activated (as indicated by elevated urine biomarkers and AVP), in the absence of a body water deficit, hyperosmolality, or thirst (Kavouras, 2019).
FLUID INTAKE REQUIREMENTS
The European Food Safety Authority (EFSA) defines adequate intake for total water as 2.5 L/d for men and 2.0 L/d for women (EFSA, 2010). According to the Institute of Medicine (IOM), the recommended total water intake is 3.7 L/d and 2.7 L/d for 19- to 50-year-old men and women, respectively, which is based on the median total water intake from the third National Health and Nutrition Examination Survey (NHANES III) (IOM, 2005). Total water intake includes water from foods (usually ~20% of total water intake) as well as beverages and plain water consumption (IOM, 2005). It is important to reiterate that guidelines for adequate intake of water represent the median value for a population. The guidelines do not intend to suggest that 3.7 L/d (men) and 2.7 L/d (women) are suitable for everyone (Sawka et al., 2005). Because of significant variation in daily water loss, both between and within individuals, there is no one-size-fits-all recommendation for daily fluid intake.
Several studies have shown that, in free-living adults, plasma osmolality is similar despite different amounts of habitual fluid consumption (IOM, 2005; Perrier et al., 2012). Thus, although ≥ 40% of US adults consume less total water than the median volume recommended by the IOM (Vieux et al., 2020), most healthy adults are not systematically hypohydrated. For some individuals, drinking less than the median value is appropriate to meet their fluid requirements (based on body size, diet, etc.), whereas others may be considered low-volume drinkers. Observations of free-living individuals report that low-volume drinkers have elevated urinary biomarkers and AVP (or copeptin) concentrations indicative of an antidiuretic challenge, in which renal water reabsorption is sufficient to prevent a TBW deficit (Perrier et al., 2013). Epidemiological data linking AVP with markers of disease have led some researchers to examine the chronic health effects of low-volume drinking. The next section provides a review of the studies aiming to address two questions at the center of this discussion: (1) Is low habitual water intake, in the absence of hypohydration, associated with adverse outcomes? and (2) Is there evidence that increasing ad libitum water intake improves health, wellness or reduces disease risk (or surrogate biomarkers where hard endpoints for disease risk were unavailable)?
LITERATURE REVIEW OF SELECTED HEALTH AND WELLNESS OUTCOMES
Urinary System
Urinary Tract Infection (UTI). UTI is a form of cystitis caused by a bacterial infection and is among the most common outpatient infections in the US. Approximately half of adult women will have at least one UTI in their life, 27% of whom will have a confirmed recurrence within 6 months. Several cross-sectional and case-control studies have investigated the effect of habitual fluid intake on UTI risk in women. Some studies (Nygaard & Linder, 1997; Vyas et al., 2015) have found that lower daily fluid intake (≤ 1.0-1.4 L/d) was associated with increased risk for UTI. Lower number of daily voids (< 3-6/d) or delayed micturition (urination) may also be risk factors for UTI (Vyas et al., 2015).
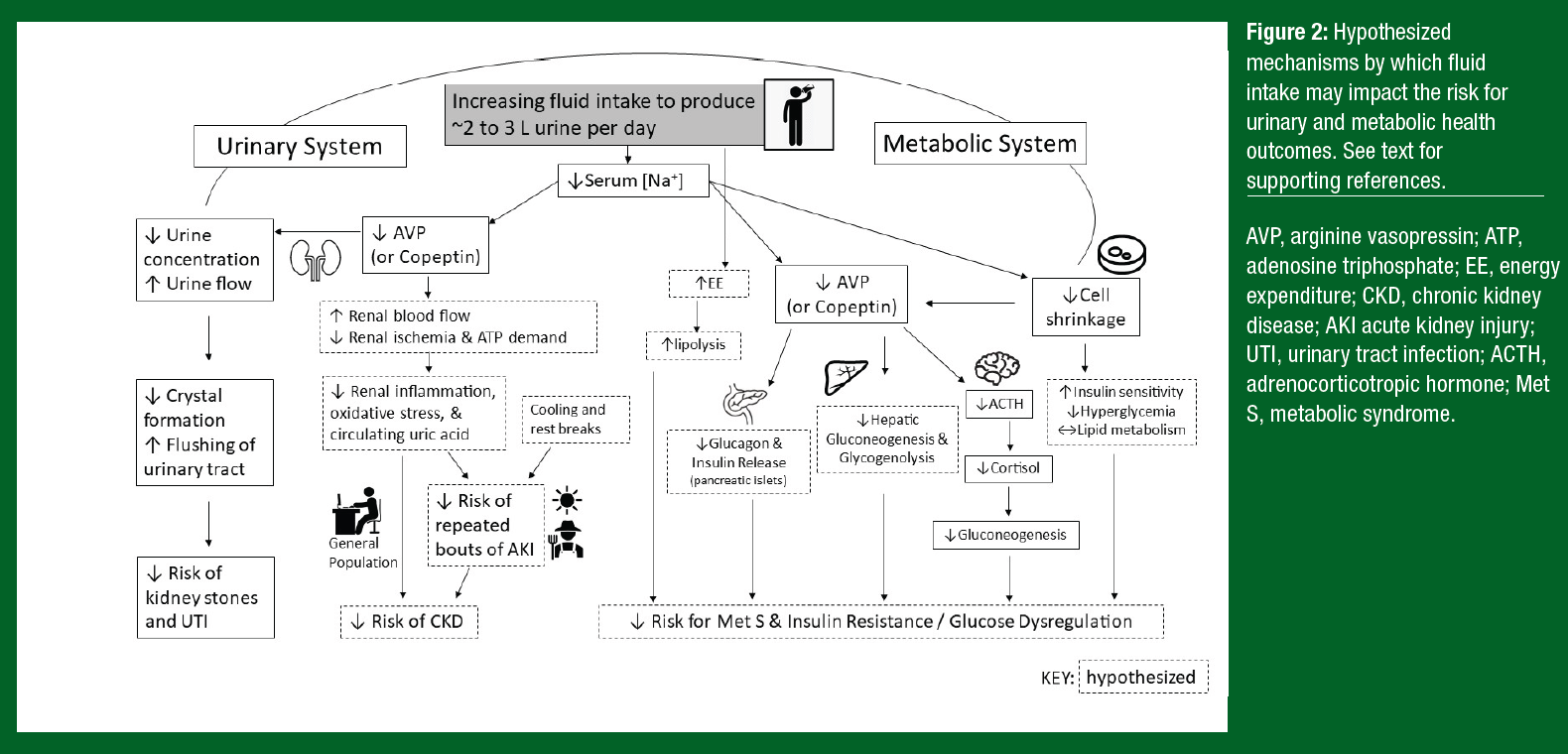
Increasing fluid intake increases urine flow, which is thought to help prevent the development of UTI by flushing the urinary tract of bacteria (Figure 2). However, until recently there was limited empirical evidence that increasing habitual fluid intake in low-volume drinkers could reduce the risk of UTI. In 2018, a randomized controlled trial (RCT) in which 140 premenopausal women suffering from recurrent cystitis were assigned to either increase their daily water intake or maintain their usual fluid intake for 12 months. There was a 48% reduction in UTI episodes after increasing total fluid intake from 1.1 to 2.8 L/d (Hooton et al., 2018). An important secondary benefit was a reduction in antibiotics used to treat UTI in the women who increased fluid intake. To date, no studies have investigated the effects of increased fluid intake volume on the risk for incident UTI.
Kidney Stones. Kidney stones are hard deposits of minerals and salts (mostly calcium oxalate mixed with calcium phosphate) that form when the urine becomes excessively supersaturated. The lifetime prevalence of kidney stones is ~9% in the US and up to 15% in other nations, and there is a trend toward an increasing burden of kidney stones over the past several decades (Romero et al., 2010). Dietary factors, including fluid intake, can influence stone formation. For example, in observational studies lower fluid intake (< 1.8 L/d) (Linder et al., 2013), lower urine output (~1.0 L/d) (Borghi et al., 1996), and higher urine osmolality (> 500-580 mOsm/kg) (Kavouras et al., 2021) have been associated with a higher risk of recurrent kidney stone formation.
There is also evidence from RCTs that increasing fluid intake in patients with a history of kidney stones can be an effective strategy in reducing the risk of recurrence (Borghi et al., 1996). Mechanistically, it is probable that urine dilution with increased fluid intake helps prevent supersaturation and crystal formation. Increased urine flow helps flush the urinary tract, which could also be helpful in preventing kidney stone formation (Figure 2). A systematic review of RCTs reported that high urine output (> 2.5 L/d) lowered long-term risk of kidney stone recurrence by ~60% (Fink et al., 2009). Accordingly, current guidelines for the secondary prevention of kidney stones recommend that stone-formers maintain a fluid intake sufficient to produce urine volume ≥ 2.5-3.0 L/d (Pearle et al., 2014; Skolarikos et al., 2015). With respect to primary prevention, higher fluid intake (> 2.0 L/d) has been associated with a lower risk of incident kidney stones in observational studies (Xu et al., 2015), but no RCTs have examined the role of fluid intake in preventing first-time stone formation.
Chronic Kidney Disease (CKD). CKD is defined as progressive loss of kidney function and has been recognized as a leading public health problem, with a total prevalence of ~13% across all stages of disease. The renal system plays vital roles in regulating fluid-electrolyte balance and filtering the blood to remove waste products via urination while retaining important nutrients. A decline in kidney function is characterized by a reduction in the estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) and leakage of protein in the urine (albuminuria).
Observational studies show that higher fluid intake (3.2 L/d) and higher urine volume (≥ 3.0 L/d) decrease the odds for renal decline among generally healthy men and women (Clark et al., 2011; Strippoli et al., 2011). It is thought that fluid intake prevents kidney function decline by suppressing AVP. Observational studies with generally healthy men and women (Clark et al., 2011; El Boustany et al., 2018) have found that higher plasma AVP or copeptin concentrations were associated with a higher risk of kidney function decline. Although increasing daily water intake can have an AVP or copeptin-lowering effect, it is unclear whether this reduces disease risk. Only one RCT has been conducted in which both AVP and markers of kidney function were measured. Despite a decrease in plasma copeptin concentrations, the 1-year decline in eGFR did not significantly differ between Stage 3 CKD patients who were coached to increase water intake by 1.0 to 1.5 L/d and patients who were asked to maintain their usual intake (Clark et al., 2018). However, intervention adherence issues may have contributed to the null effect, as urine output in the intervention group was only 0.6 L/d higher than the control group. More research is needed to demonstrate a causal link between chronic underhydration, AVP, and CKD.
Several reports over the last decade have described an epidemic of CKD occurring along the Pacific coast of Central America. CKD is a leading cause of death in this region among working-age men in lower-altitude communities (e.g., agriculture workers, sugarcane harvesters, brickmaking workers). This epidemic, termed Mesoamerican nephropathy, is thought to be caused by recurrent acute kidney injury (AKI) from repeated exposure to extreme heat stress, strenuous physical work, and hypohydration (Weiner et al., 2013).
The potential mechanisms by which fluid intake may impact the risk of CKD are illustrated in Figure 2. In brief, exercise and heat stress cause redistribution of blood flow away from the renal cortex, which can impair oxygen delivery and in turn limit adenosine triphosphate (ATP) production needed for normal cell functioning. Hypohydration exacerbates the risk of renal injury because of the increased ATP demand for water and electrolyte conservation. Collectively, these factors may lead to a cascade of events that promote inflammation and oxidative stress, thereby increasing the risk of glomerular sclerosis and a reduction in the number of functioning nephrons (Chapman et al., 2021). It is important to note, however, that proposed mechanisms are largely derived from animal data. More research is needed to establish direct support for recurrent heat-related AKI in the etiology of CKD in manual laborers.
Metabolism
Metabolic Syndrome (MetS). MetS refers to the co-occurrence of several conditions that increase the risk for cardiovascular disease, type 2 diabetes mellitus (T2D), and stroke, including insulin resistance, obesity, dyslipidemia, and hypertension (Huang, 2009). Chronic hypohydration has been associated with increased risk for MetS and its components. For example, potential indicators of hypohydration, such as serum sodium, spot urine volume, and osmolality, have been associated with the prevalence of obesity, high waist circumference, insulin resistance, low levels of high-density lipoprotein (HDL), hypertension, and metabolic syndrome (Stookey et al., 2020). Similarly, a RCT found that experimentally induced hypohydration (-1.6% body mass loss) acutely impaired the blood glucose response to 3 days of low total water intake (plasma osmolality ~298 mOsm/kg) in people with T2D (Johnson et al., 2017), although this result may not translate to healthy volunteers (Carroll et al., 2019). Further, another RCT in healthy adults found no changes in fasting blood glucose or AVP concentrations with an additional 1.1 L/d of water for 12 weeks (Nakamura et al., 2020).
Although RCTs have not elucidated the effects of fluid intake on MetS, underlying mechanisms supporting the aforementioned associations have been thoroughly investigated. When AVP is habitually activated to maintain plasma osmolality, it may lead to negative effects on glucose regulation and diabetes. Negative effects of chronic AVP secretion on glucose regulation may stem from AVP’s stimulation of hepatic gluconeogenesis and glycogenolysis through the vasopressin receptor 1A (V1aR) (Keppens & de Wulf, 1979; Whitton et al., 1978) and release of both glucagon and insulin through vasopressin receptor 1b (V1bR) in the pancreatic islets (Abu-Basha et al., 2002). Adrenocorticotropic hormone (ACTH) is released following V1bR activation in the anterior pituitary gland via AVP. This action elevates adrenal cortisol secretion leading to gluconeogenesis, which over time can be detrimental and undesirable for a variety of organ systems (Perrier et al., 2021). Further, increases in serum sodium concentration promote cell shrinkage, potentially predisposing individuals to obesity through physiological responses such as the upregulation of proteolysis and glycogenolysis, which can trigger insulin resistance (Lang et al., 1998) (Figure 2).
In support of these mechanistic studies, high plasma copeptin concentrations have been associated with insulin resistance, diabetes mellitus, and MetS (Enhörning et al., 2010; Saleem et al., 2009). Others have also demonstrated a link between copeptin, diabetes mellitus, and obesity but not the entire MetS cluster (Enhörning et al., 2013; Then et al., 2015). However, because these studies are mostly cross-sectional, the findings need to be reproduced in prospective studies and ideally RCTs.
The role of hydration in obesity and subsequent MetS may also stem from the effects of fluid intake on energy expenditure (EE) and weight loss. Some evidence suggests that ingestion of ~500 mL of water increases EE, with a small contribution to increased thermogenesis from heating the water to body temperature (Boschmann et al., 2003). However, when others attempted to replicate these results, EE did not increase after drinking distilled water or saline solution (Brown et al., 2006). Early studies also displayed mixed results in changes in EE when using water as a control to note changes in EE following a caloric intervention. More recently, a sham controlled RCT showed drinking purified water (500 mL) led to only small increases in EE that were not different from sham controls (Charrière et al., 2015). The authors concluded that drinking purified water does not result in thermogenesis or fat oxidation. Inconsistencies in EE reports may be reflective of methodological differences or measurement limitations.
Although the effect of water on EE appears minimal, intervention studies suggest that when water was consumed prior to each meal, this led to greater weight loss than a hypocaloric diet alone (Dennis et al., 2010). Additionally, drinking more than one liter of water per day was associated with a decreased risk for becoming overweight in a Chinese population. The authors reported that every cup of water drank was associated with a 6.5% (men) and 8.4% (women) decrease in the risk for becoming overweight (Pan et al., 2020). However, the effect of consuming plain water on diet pattern and total caloric intake is inconsistent across trials (Kant & Graubard, 2018). It should be noted that replacing caloric beverages with water may be a potential weight loss strategy (Tate et al., 2012). While these results suggest plain water intake as a potential strategy for the prevention of weight gain or increasing the amount of weight lost, these studies have several limitations.
Cardiovascular System
Cardiovascular Disease (CVD). CVD is a group of disorders of the heart and blood vessels and is the number one cause of morbidity and mortality in the United States (Virani et al., 2020) and the leading cause of death globally (WHO, 2021). Consequently, practical and low-cost ways to reduce risk factors for CVD and related conditions are of public health interest. Hypohydration has been linked to increased risk for cardiovascular risk factors and associated conditions in cross-sectional studies; however, RCTs are limited. Preclinical research suggests that hypohydration may impair endothelial function, increase sympathetic nervous activity, and exacerbate orthostatic intolerance, contributing to impaired blood pressure regulation and vascular function (Watso & Farquhar, 2019). Further, hypohydration stimulates AVP and subsequent glucocorticoid release, which upregulates serum and glucocorticoid-inducible kinase 1, potentially encouraging the development of hypertension, obesity, diabetes, thrombosis, stroke, and cardiac fibrosis (Lang et al., 2017).
In support of this, one prospective cohort study demonstrated that increasing plain water intake decreased the risk of cardiovascular disease (Chan et al., 2002), but this analysis was conducted in California Seventh-day Adventist households, making it difficult to generalize to other populations. Contrastingly, additional prospective studies did not observe an association between water or total fluid intake and ischemic heart disease, stroke, all-cause mortality, or cardiovascular mortality (Kant & Graubard, 2017; Palmer et al., 2014).
Orthostatic Intolerance. Orthostatic tolerance is the ability to maintain upright posture against gravity through maintenance of cerebral perfusion, preventing syncope (i.e., fainting, or transient loss of consciousness usually leading to falling). Syncope occurs at least once in 22% of the population, and 9% of these individuals have recurrent episodes (Chen et al., 2003). Clinically, orthostatic (postural) hypotension is defined as a fall in blood pressure of over 20 mmHg systolic (or 10 mmHg diastolic) upon standing (Kaufmann, 1996) or during head-up tilt to at least 60°. Head-up tilting and lower body negative pressure are common experimental methods used to assess tolerance to orthostatic stressors. Orthostatic hypotension may result from a variety of diseases or medications but may also result from non-neurogenic causes, which include blood volume depletion, vasodilation, and cardiac impairment (Mathias & Kimber, 1998). Nonpharmacological treatments of orthostatic hypotension include encouraging a high intake of salt (from 6 to 9 g sodium chloride/d) and fluid (2-3 L/d) for chronic expansion of the intravascular volume (Arnold & Shibao, 2013).
An acute bout of exercise impairs orthostatic tolerance (Eichna et al., 1947), and even low levels of hypohydration may contribute to orthostatic intolerance (Davis & Fortney, 1997). However, an early observational study did not demonstrate differences in hydration status, as measured by percent body mass loss and plasma volume, between individuals that collapsed and those that did not following an ultra-endurance race (Holtzhausen & Noakes, 1995). Although there are some extreme cases where postexercise hypotension can become symptomatic, most postexercise syncope is likely neurally mediated (Halliwill et al., 2014). Water ingestion is suggested as a countermeasure against postexercise syncope (Krediet et al., 2004). However, this is mostly due to the effect of water intake on the pressor response, instead of its effect on hydration status (Halliwill et al., 2014). In agreement with this, water intake (~500 mL) (Davis & Fortney, 1997; Jordan et al., 2000; Schroeder et al., 2002) improved experimental measures of orthostatic tolerance (heart rate, blood pressure, tachycardia, stroke volume, head-up tilt), but not all studies reported this benefit in healthy young individuals (Jordan et al., 2000).
Gastrointestinal System
One of the most common gastrointestinal disorders worldwide is chronic idiopathic constipation, with a median prevalence of ~16%. Cross-sectional studies suggest that low fluid intake may be associated with constipation in adults (≤ 1.0-1.8 L/d) (Yurtdas et al., 2020) as well as children and adolescents (~0.4-1.0 L/d) (Boilesen et al., 2017). Additionally, an intervention study found that one week of fluid deprivation (0.5 vs. 2.5 L/d the week prior) was associated with lower stool frequency and stool weight in healthy men (Klauser et al., 1990).
There is limited evidence, however, that increasing fluid intake in low-volume drinkers affects stool output. One RCT found that increased fluid intake (1.0 to 2.1 L/d) enhanced the effect of a high-fiber diet on stool frequency in men and women with functional constipation (Anti et al., 1998). But other RCTs found no effect of increased water intake (1.0-1.2 to 1.6-1.8 L/d) and/or urine output (~1.0 to 1.5-2.0 L/d) on stool weight or frequency in healthy men and women (Chung et al., 1999; Ziegenhagen et al., 1991). Thus, while overt dehydration of the colonic contents can harden stools, increasing fluid intake may not improve stool consistency as most ingested water is absorbed in the intestines and subsequently any excess excreted via the kidneys.
Neurological System
Headache. Recurrent headaches are among the most common disorders of the nervous system, affecting approximately half of the adult population globally. Tension-type headache is the most prevalent headache disorder (42%), followed by migraine (11%) and chronic daily headache (3%). Women are more likely to experience headache disorders, especially migraines. Headaches are not only painful, but one of the leading causes of years lost due to disability.
Some fluid restriction studies suggest that 1.4-2.7% body mass deficit accrued over 8-37 h increases headache symptoms (Armstrong et al., 2012; Shirreffs et al., 2004). In addition, one cross-sectional study in women found lower ratings of migraine disability, pain severity, and headache frequency and duration in individuals with higher water intake (2.1-2.3 L/d) versus low water drinkers (1.6-1.9 L/d) (Khorsha et al., 2020). While fluid restriction may be a risk factor for headache, there are limited data on the effect of increased fluid intake for the prevention or treatment of headache in patients with recurrent headaches. One small RCT found significant improvement in subjective measures (Migraine-Specific Quality of Life questionnaire) when male and female patients increased water intake from 1.7 to 2.6 L/d for 3 months, but there was no effect on objective measures, including the number of headache days or medication use (Spigt et al., 2012). More research is needed to elucidate the role of hydration in headache disorders as well as the potential mechanisms of action by which fluid intake could prevent or treat symptoms.
Cognitive Performance. The effect of hypohydration on cognitive performance has been discussed in a previous Sports Science Exchange article (Wittbrodt & Barnes, 2020). In brief, mild hypohydration (≥ 1% body mass deficit) may have a small but significant detrimental effect on overall cognitive performance. Cognitive impairments are more likely with higher levels of hypohydration (> 2% body mass deficit). In addition, cognitive impairments are greater in higher-order domains (executive function and attention) than simple reaction time–based tests (Wittbrodt & Millard-Stafford, 2018).
There is limited information available on the effects of experimentally increased fluid intake above habitual intake. Some studies have investigated the acute effects of 120-500 mL water ingestion, with mixed results on cognitive performance measured 2 to 50 minutes after ingestion. However, effects may be modified by the level of thirst sensation; that is, performance improvements were more likely in subjects with high subjective thirst ratings at the time of water ingestion (Edmonds et al., 2013b; Rogers et al., 2001).
Mood. Like cognitive performance, mood can have a significant impact on day-to-day functioning and is an important aspect of overall wellness. Research has consistently shown that short-term hypohydration (1.1-2.7% body mass deficit) induced via acute fluid restriction decreases feelings of energy, vigor, alertness, and ability to concentrate and/or increases fatigue, tiredness, tension, and anxiety (Armstrong et al., 2012; Pross et al., 2014; Shirreffs et al., 2004; Szinnai et al., 2005).
Less is known about the effect of increasing fluid ingestion above baseline or habitual intake. Small volumes of water supplementation (120-500 mL) may have short-term (~2-20 min) benefits on alertness, but no effect on mood ~25 to 50 minutes after ingestion (Edmonds, et al., 2013a; Rogers et al., 2001). In addition, one study reported decreased feelings of fatigue, but no impact on alertness after 3 days of increased water intake (1.0 to 2.5 L/d) in habitually low drinkers (Pross et al., 2014). The positive effects of water supplementation on mood may be a result of the oropharyngeal reflex inhibition of thirst (Figaro & Mack, 1997) and/or the transient increase in sympathetic activity and plasma norepinephrine with or without increases in blood pressure shortly after drinking in healthy subjects (Schroeder et al., 2002; Scott et al., 2001).
OTHER CONSIDERATIONS
Fluid Composition
The USDA and EFSA recommendations for water intake include water from other beverages and foods, as all sources of water contribute to the maintenance of water balance. Studies have shown similar hydration status after only plain water was consumed compared with the same total volume of fluid from a combination of water and other beverages with or without caffeine or calories. For instance, 24-h urine volume, osmolality, and color did not differ after 1.7 L/d ingestion of plain water versus 1.7 L/d of various combinations of water, cola, diet cola, and orange juice in free-living, healthy adult men (Tucker et al., 2015).
Regarding health outcomes, most studies have reported total fluid intake and did not clearly distinguish between plain water and water from other beverages. An observational study found an association between ≥ 1 serving of sugar-sweetened beverages (cola and non-cola) per day and increased risk for kidney stone formation (Ferraro et al., 2013). With respect to chronic renal health, a cross-sectional analysis of NHANES data found an association between consumption of ≥ 2 sugar-sweetened soft drinks/d and albuminuria (a marker for CKD). On the other hand, there was no association between ≤ 1 soft drinks or ≥ 2 diet sodas/d and albuminuria (Shoham et al., 2008). Additional studies are needed to determine whether confounding effects due to diet or lifestyle factors are at play. It is thought that fructose in sugar-sweetened beverages may impact renal health through augmentation of the fructokinase pathway and AVP release, but this hypothesis is based on experimental data in animal models. RCTs are needed to establish a causal relationship between specific beverage types/amounts and health outcomes of the urinary system. With respect to metabolic health, it is important to note that habitual replacement of caloric beverages with water can contribute to body weight loss (Tate et al., 2012). Thus, taken together, it stands to reason that for individuals on weight management programs or at risk for cardiometabolic or renal disease, increases in daily fluid intake should come mostly from plain water.
Safety and Feasibility
It is important to note potential risks or side effects from increased water intake. Overdrinking plain water or other low-sodium fluid could lead to the dilution of blood sodium concentration (hyponatremia). This condition is potentially dangerous, as severe hyponatremia (< 125 mmol/L) can lead to edema, cardiorespiratory arrest, coma, and death. In particular, individuals with reduced renal capacity to excrete free water (e.g., the elderly, individuals with inappropriate AVP secretion, dialysis patients) may be at risk for negative consequences of fluid overload. However, severe hyponatremia is rare in the general population and usually occurs because of overzealous fluid intake (IOM, 2005). Moreover, intervention studies have reported minimal differences in adverse events, drop-out rates, or serum sodium concentration between groups that increased water intake compared with no change in habitual intake (Clark et al., 2018; Hooton et al., 2018; Nakamura et al., 2020). This is probably because the premise of recommendations around hydration for health is to increase water intake in low-volume drinkers. Increased habitual water intake is not necessary for individuals already drinking adequately and producing 2-3 L of urine/d.
A common issue reported in intervention studies is an inability of some study participants to maintain the desired increase in daily water intake. Where subject compliance was assessed, 24-h fluid intake (or urine output) in the intervention group was often lower than prescribed (Chung et al., 1999; Clark et al., 2018; Spigt et al., 2012). The reason for inconsistent adherence is unclear but could be related to a waning desire or feasibility to maintain long-term increased water intake. Other practical drawbacks with increased water intake include frequent need for urination, which may pose a problem if someone has irregular or unpredictable access to a clean bathroom (e.g., occupational settings). At any rate, suboptimal compliance with study instructions to increase water intake represents a major limitation in RCTs aiming to determine effects on health outcomes.
LIMITATIONS AND FUTURE DIRECTIONS
Currently, the effects of habitual fluid intake or hydration status are suggestive of benefit for some chronic diseases, including CKD, but for other areas, such as MetS and CVD, the benefit is less clear. Some of the more common limitations include study design and validity and reproducibility of hydration status measures. Observational study designs make up the bulk of the evidence base and present major limitations. Here, the most profound limitation is the potential effect of confounding, where an unmeasured or poorly measured factor may falsely obscure or reveal an association between an exposure and outcome. Measurement error in observational studies can bias effect estimates in unpredictable ways but tends to bias estimates toward the null or weaker effects. Further, in many instances the literature was restricted to cross-sectional studies, where no temporal relationship can be established, making interpretation of results difficult. Such studies are best reserved for hypothesis generating and should always be examined in prospective observational studies, or ideally RCTs, though many of the health outcomes of interest are not well-suited for RCTs (e.g., rare and extended latency between exposure and disease).
Validity and reproducibility of measurements were also major limitations for both the observational and interventional studies reviewed. Definitions, terms, and biomarkers used for describing and measuring hydration status or health conditions are inconsistent throughout the literature. Other key measurements, such as overall fluid consumption patterns or estimations of fluid intake, were not always examined or reported. Further, many studies reviewed were limited to highly specific populations and may lack generalizability to other populations. Where possible, future studies should employ RCTs with consistent, serial measures of hydration status and records of subject compliance. Future observational studies should focus on including a broad set of potential confounders and use serial and validated measures of hydration status.
CONCLUSION
Appropriate hydration habits represent a simple and inexpensive method to potentially impact important health and wellness outcomes. For at-risk individuals, there is evidence that increasing daily fluid intake can decrease the recurrence of UTIs and kidney stones. In addition, water ingestion may acutely enhance orthostatic tolerance, which may be beneficial to individuals susceptible to syncope after exercise. For weight management, habitual replacement of caloric beverages with water can contribute to weight loss. On the other hand, fluid restriction consistently impairs cognitive performance and mood and may also be a risk factor for headache and functional constipation. However, aside from UTIs and kidney stones, there is limited evidence that increasing habitual fluid intake in low-volume drinkers can prevent or treat health outcomes. RCTs are needed to establish causal links between fluid intake and CKD, metabolic syndrome, and cardiovascular disease.
PRACTICAL APPLICATIONS
- Fluid restriction leading to significant hypohydration (> 1-2% body mass deficit) should be avoided since this may be associated with decrements in mood (e.g., feelings of energy, vigor, alertness, and ability to concentrate) and cognition (particularly executive function domains) as well as increased risk for headache and functional constipation.
- Increasing habitual fluid intake can decrease the risk for recurrent kidney stones and UTI.
- Guidelines for adequate intake of water are not intended to be one-size-fits-all recommendations because optimal daily fluid intake varies among individuals depending on diet, metabolism, transcutaneous water vapor loss, and sweating, among other factors.
- Instead, it has been proposed that total fluid intake should be sufficient to produce 2-3 L of urine/d. Urinary markers (≥ 5-7 voids/d, < 3-4 on color chart, ≤ 500 mOsm/kg) may be used to monitor and adjust fluid intake accordingly.
- Additional fluid volumes should largely come from plain water, especially those on weight management programs or at risk for cardiometabolic or renal disease.
ACKNOWLEDGMENTS
The authors would like to thank Megan Engel and David Keyes for assistance in the preparation of the manuscript. The authors are employed by PepsiCo R&D. The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Abu-Basha, E.A., S. Yibchok-Anun, and W.H. Hsu (2002). Glucose dependency of arginine vasopressin-induced insulin and glucagon release from the perfused rat pancreas. Metabolism 51:1184-1190.
Anti, M., G. Pignataro, A. Armuzzi, A. Valenti, E. Iascone, R. Marmo, A. Lamazza, A.R. Pretaroli, V. Pace, P. Leo, A. Castelli, and G. Gasbarrini (1998). Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterol. 45:727-732.
Armstrong, L.E., M.S. Ganio, D.J. Casa, E.C. Lee, B.P. McDermott, J.F. Klau, L. Jimenez, L. Le Bellego, E. Chevillotte, and H.R. Lieberman (2012). Mild dehydration affects mood in healthy young women. J. Nutr. 142:382-388.
Arnold, A.C., and C. Shibao (2013). Current concepts in orthostatic hypotension management. Curr. Hypertens. Rep. 15:304-312.
Boilesen, S.N., S. Tahan, F.C. Dias, L. Melli, and M.B. de Morais (2017). Water and fluid intake in the prevention and treatment of functional constipation in children and adolescents: is there evidence? J. Pediatr. 93:320-327.
Borghi, L., T. Meschi, F. Amato, A. Briganti, A. Novarini, and A. Giannini (1996). Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J. Urol. 155:839-843.
Boschmann, M., J. Steiniger, U. Hille, J. Tank, F. Adams, A.M. Sharma, S. Klaus, F.C. Luft, and J. Jordan (2003). Water-induced thermogenesis. J. Clin. Endocrinol. Metab. 88:6015-6019.
Brown, C.M., A.G. Dulloo, and J.P. Montani (2006). Water-induced thermogenesis reconsidered: the effects of osmolality and water temperature on energy expenditure after drinking. J. Clin. Endocrinol. Metab. 91:3598-3602.
Carroll, H.A., I. Templeman, Y.C. Chen, R.M. Edinburgh, E.K. Burch, J.T. Jewitt, G. Povey, T.D. Robinson, W.L. Dooley, R. Jones, K. Tsintzas, W. Gallo, O. Melander, D. Thompson, L.J. James, L. Johnson, and J.A. Betts (2019). Effect of acute hypohydration on glycemic regulation in healthy adults: a randomized crossover trial. J. Appl. Physiol. 126:422-430.
Chan, J., S.F. Knutsen, G.G. Blix, J.W. Lee, and G.E. Fraser (2002). Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. Am. J. Epidemiol. 155:827-833.
Chapman, C.L., B.D. Johnson, M.D. Parker, D. Hostler, R.R. Pryor, and Z. Schlader (2021). Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 8:108-159.
Charrière, N., J.L. Miles-Chan, J.P. Montani, and A.G. Dulloo (2015). Water-induced thermogenesis and fat oxidation: a reassessment. Nutr. Diab. 5:e190.
Chen, L.Y., B.J. Gersh, D.O. Hodge, W. Wieling, S.C. Hammill, and W.K. Shen (2003). Prevalence and clinical outcomes of patients with multiple potential causes of syncope. Mayo Clin. Proc. 78:414-420.
Cheuvront, S.N., and R.W. Kenefick (2016). Am I drinking enough? Yes, mo, and maybe. J. Am. Coll. Nutr. 35:185-192.
Chung, B.D., U. Parekh, and J.H. Sellin (1999). Effect of increased fluid intake on stool output in normal healthy volunteers. J. Clin. Gastroenterol. 28:29-32.
Clark, W.F., J.M. Sontrop, J.J. Macnab, R.S. Suri, L. Moist, M. Salvadori, and A.X. Garg (2011). Urine volume and change in estimated GFR in a community-based cohort study. Clin. J. Am. Soc. Nephrol. 6:2634-2641.
Clark, W.F., J.M. Sontrop, S.H. Huang, K. Gallo, L. Moist, A.A. House, M.S. Cuerden, M.A. Weir, A. Bagga, S. Brimble, A. Burke, N. Muirhead, S. Pandeya, and A.X. Garg (2018). Effect of coaching to increase water intake on kidney function decline in adults with chronic kidney disease: The CKD WIT randomized clinical trial. J. Am. Med. Assoc. 319:1870-1879.
Davis, J.E., and S.M. Fortney (1997). Effect of fluid ingestion on orthostatic responses following acute exercise. Int. J. Sports Med. 18:174-178.
Dennis, E.A., A.L. Dengo, D.L. Comber, K.D. Flack, J. Savla, K.P. Davy, and B.M. Davy (2010). Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity 18:300-307.
Edmonds, C.J., R. Crombie, H. Ballieux, M.R. Gardner, and L. Dawkins (2013a). Water consumption, not expectancies about water consumption, affects cognitive performance in adults. Appetite 60:148-153.
Edmonds, C.J., R. Crombie, and M.R. Gardner (2013b). Subjective thirst moderates changes in speed of responding associated with water consumption. Front. Hum. Neurosci. 7:363.
EFSA. (2010). Scientific opinion on dietary reference values for water. EFSA J. 8:1459-1507.
Eichna, L.W., S.M. Horvath, and W.B. Bean (1947). Post-exertional orthostatic hypotension. Am. J. Med. Sci. 213: 641-654.
El Boustany, R., I. Tasevska, E. Meijer, L.M. Kieneker, S. Enhorning, G. Lefevre, K. Mohammedi, M. Marre, F. Fumeron, B. Balkau, N. Bouby, L. Bankir, S.J. Bakker, R. Roussel, O. Melander, R.T. Gansevoort, and G. Velho (2018). Plasma copeptin and chronic kidney disease risk in 3 European cohorts from the general population. JCI Insight 3:121479.
Enhörning, S., T.J. Wang, P.M. Nilsson, P. Almgren, B. Hedblad, G. Berglund, J. Struck, N.G. Morgenthaler, A. Bergmann, E. Lindholm, L. Groop, V. Lyssenko, M. Orho-Melander, C. Newton-Cheh, and O. Melander (2010). Plasma copeptin and the risk of diabetes mellitus. Circulation 121:2102-2108.
Enhörning, S., L. Bankir, N. Bouby, J. Struck, B. Hedblad, M. Persson, N.G. Morgenthaler, P.M. Nilsson, and O. Melander (2013). Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int. J. Obes. 37:598-603.
Ferraro, P.M., E.N. Taylor, G. Gambaro, and G.C. Curhan (2013). Soda and other beverages and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 8:1389-1395.
Figaro, M.K., and G.W. Mack (1997). Regulation of fluid intake in dehydrated humans: role of oropharyngeal stimulation. Am. J. Physiol. 272:R1740-R1746.
Fink, H.A., J.W. Akornor, P.S. Garimella, R. MacDonald, A. Cutting, I.R. Rutks, M. Monga, and T.J. Wilt (2009). Diet, fluid, or supplements for secondary prevention of nephrolithiasis: a systematic review and meta-analysis of randomized trials. Eur. Urol. 56:72-80.
Halliwill, J.R., D.C. Sieck, S.A. Romero, T.M. Buck, and M.R. Ely (2014). Blood pressure regulation X: what happens when the muscle pump is lost? Post-exercise hypotension and syncope. Eur. J. Appl. Physiol. 114:561-578.
Holtzhausen, L.M., and T.D. Noakes (1995). The prevalence and significance of post-exercise (postural) hypotension in ultramarathon runners. Med. Sci. Sports Exerc. 27:1595-1601.
Hooton, T.M., M. Vecchio, A. Iroz, I. Tack, Q. Dornic, I. Seksek, and Y Lotan (2018). Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: A randomized clinical trial. J. Am. Med. Assoc. Intern. Med. 178:1509-1515.
Huang, P.L. (2009). A comprehensive definition for metabolic syndrome. Disease models & mechanisms. Dis. Model Mech. 2:231-237.
IOM (2005). Water. In: Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. National Academies Press, pp.73-185.
Johnson, E.C., C.N. Bardis, L.T. Jansen, J.D. Adams, T.W. Kirkland, and S.A. Kavouras (2017). Reduced water intake deteriorates glucose regulation in patients with type 2 diabetes. Nutr. Res. 43:25-32.
Jordan, J., J.R. Shannon, B.K. Black, Y. Ali, M. Farley, F. Costa, A. Diedrich, R.M. Robertson, I. Biaggioni, and D. Robertson (2000). The pressor response to water drinking in humans : a sympathetic reflex? Circulation 101:504-509.
Kant, A.K., and B.I. Graubard (2017). A prospective study of water intake and subsequent risk of all-cause mortality in a national cohort. Am. J. Clin. Nutr. 105:212-220.
Kant, A.K., and B.I. Graubard (2018). Complementary and compensatory dietary changes associated with consumption or omission of plain water by US adults. Appetite 128:255-262.
Kaufmann, H. (1996). Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin. Auton. Res. 6:125-126.
Kavouras, S.A. (2019). Hydration, dehydration, underhydration, optimal hydration: Are we barking up the wrong tree? Eur. J. Nutr. 58:471-473.
Kavouras, S.A., H.G. Suh, M. Vallet, M. Daudon, A. Mauromoustakos, M. Vecchio, and I. Tack (2021). Urine osmolality predicts calcium-oxalate crystallization risk in patients with recurrent urolithiasis. Urolithiasis 49:399-405.
Keppens, S., and H. de Wulf (1979). The nature of the hepatic receptors involved in vasopressin-induced glycogenolysis. Biochim. Biophys. Acta 588:63-69.
Khorsha, F., A. Mirzababaei, M. Togha, and K. Mirzaei (2020). Association of drinking water and migraine headache severity. J. Clin. Neurosci. 77:81-84.
Klauser, A.G., A. Beck, N.E. Schindlbeck, and S.A. Muller-Lissner (1990). Low fluid intake lowers stool output in healthy male volunteers. Z. Gastroenterol. 28:606-609.
Krediet, C.T.P., A.A.M. Wilde, W. Wieling, and J.R. Halliwill (2004). Exercise related syncope, when it’s not the heart. Clin. Auton. Res. 14:i25-i36.
Lang, F., G.L. Busch, M. Ritter, H. Völkl, S. Waldegger, E. Gulbins, and D. Häussinger (1998). Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78:247-306.
Lang, F., I. Guelinckx, G. Lemetais, and O. Melander (2017). Two liters a day keep the doctor away? Considerations on the pathophysiology of suboptimal fluid intake in the common population. Kidney Blood Press. Res. 42:483-494.
Linder, B.J., L.J. Rangel, and A.E. Krambeck (2013). The effect of work location on urolithiasis in health care professionals. Urolithiasis 41:327-331.
Mathias, C.J., and J.R. Kimber (1998). Treatment of postural hypotension. J. Neurol. Neurosurg. Psych. 65:285-289.
Nakamura, Y., H. Watanabe, A. Tanaka, M. Yasui, J. Nishihira, and N. Murayama, N. (2020). Effect of increased daily water intake and hydration on health in japanese adults. Nutrients 12:1191.
Nygaard, I., and M. Linder (1997). Thirst at work--an occupational hazard? Int. Urogynecol. J. Pelvic Floor Dysfunct. 8:340-343.
Palmer, S.C., G. Wong, S. Iff, J. Yang, V. Jayaswal, J.C. Craig, E. Rochtchina, P. Mitchell, J.J. Wang, and G.F. Strippoli (2014). Fluid intake and all-cause mortality, cardiovascular mortality and kidney function: a population-based longitudinal cohort study. Nephrol. Dial. Transplant 29:1377-1384.
Pan, X.B., H.J. Wang, B. Zhang, Y.L. Liu, S.F. Qi, and Q.B. Tian (2020). Plain water intake and association with the risk of overweight in the chinese adult population: China Health and Nutrition Survey 2006-2011. J. Epidemiol. 30:128-135.
Pearle, M.S., D.S. Goldfarb, D.G. Assimos, G. Curhan, C.J. Denu-Ciocca, B.R. Matlaga, M. Monga, K. Penniston, G.M. Preminger, T.M. Turk, J.R. White, and American Urological Association (2014). Medical management of kidney stones: AUA guideline. J. Urol. 192:316-324.
Perrier, E.T. (2017). Shifting focus: From hydration for performance to hydration for health. Ann. Nutr. Metab. 70 (Suppl 1):4-12.
Perrier, E., S. Vergne, A. Klein, M. Poupin, P. Rondeau, L. Le Bellego, L.E. Armstrong, F. Lang, J. Stookey, and I. Tack (2013). Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. .J Nutr. 109:1678-1687.
Perrier, E.T., L.E. Armstrong, J.H. Bottin, W.F. Clark, A. Dolci, I. Guelinckx, A. Iroz, S.A. Kavouras, F. Lang, H.R. Lieberman, O. Melander, C. Morin, I. Seksek, J.D. Stookey, I. Tack, T. Vanhaecke, M. Vecchio, and F. Péronnet (2021). Hydration for health hypothesis: a narrative review of supporting evidence. Eur. J. Nutr. 60:1167-1180.
Pross, N., A. Demazieres, N. Girard, R. Barnouin, D. Metzger, A. Klein, E. Perrier, and I. Guelinckx (2014). Effects of changes in water intake on mood of high and low drinkers. PLoS One 9:e94754.
Robertson, G.L. (1984). Abnormalities of thirst regulation. Kidney Int. 25:460-469.
Rogers, P.J., A. Kainth, and H.J. Smit (2001). A drink of water can improve or impair mental performance depending on small differences in thirst. Appetite 36:57-58.
Romero, V., H. Akpinar, and D.G. Assimos (2010). Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 12:e86-e96.
Saleem, U., M. Khaleghi, N.G. Morgenthaler, A. Bergmann, J. Struck, T.H. Mosley, and I.J. Kullo (2009). Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J. Clin. Endocrinol. Metab. 94:2558-2564.
Sawka, M.N., S.N. Cheuvront, and R. Carter (2005). Human water needs. Nutr. Rev. 63:S30-S39.
Schroeder, C., V.E. Bush, L.J. Norcliffe, F.C. Luft, J. Tank, J. Jordan, and R. Hainsworth (2002). Water drinking acutely improves orthostatic tolerance in healthy subjects. Circulation 106:2806-2811.
Scott, E.M., J.P. Greenwood, S.G. Gilbey, J.B. Stoker, and D.A. Mary (2001). Water ingestion increases sympathetic vasoconstrictor discharge in normal human subjects. Clin. Sci. 100:335-342.
Shirreffs, S.M., S.J. Merson, S.M. Fraser, and D.T. Archer (2004). The effects of fluid restriction on hydration status and subjective feelings in man. Br. J. Nutr. 91:951-958.
Shoham, D.A., R. Durazo-Arvizu, H. Kramer, A. Luke, S. Vupputuri, A. Kshirsagar, and R.S. Cooper (2008). Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999-2004. PLoS One 3:e3431.
Skolarikos, A., M. Straub, T. Knoll, K. Sarica, C. Seitz, A. Petrik, and C. Turk (2015). Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur. Urol. 67:750-763.
Spigt, M., N. Weerkamp, J. Troost, C.P. van Schayck, and J.A. Knottnerus (2012). A randomized trial on the effects of regular water intake in patients with recurrent headaches. Fam. Pract. 29:370-375.
Stookey, J.D., S. Kavouras, H. Suh, and F. Lang (2020). Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 years in the U.S. population aged 51-70 years. Nutrients 12:905.
Strippoli, G.F., J.C. Craig, E. Rochtchina, V.M. Flood, J.J. Wang, and P. Mitchell (2011). Fluid and nutrient intake and risk of chronic kidney disease. Nephrology 16:326-334.
Szinnai, G., H. Schachinger, M.J. Arnaud, L. Linder, and U. Keller (2005). Effect of water deprivation on cognitive-motor performance in healthy men and women. Am. J. Physiol. 289:R275-R280.
Tate, D.F., G. Turner-McGrievy, E. Lyons, J. Stevens, K. Erickson, K. Polzien, M. Diamond, X. Wang, and B. Popkin (2012). Replacing caloric beverages with water or diet beverages for weight loss in adults: Main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 95:555-563.
Then, C., B. Kowall, A. Lechner, C. Meisinger, M. Heier, W. Koenig, A. Peters, W. Rathmann, and J. Seissler (2015). Plasma copeptin is associated with type 2 diabetes in men but not in women in the population-based KORA F4 study. Acta Diabetol. 52:103-112.
Tucker, M.A., M.S. Ganio, J.D. Adams, L.A. Brown, C.B. Ridings, J.M. Burchfield, F.B. Robinson, J.L. McDermott, B.A. Schreiber, N.E. Moyen, T.A. Washington, A.C. Bermudez, M.P. Bennett, and M.E. Buyckx (2015). Hydration status over 24-h is not affected by ingested beverage composition. J. Am. Coll. Nutr. 34:318-327.
Virani, S.S., A. Alonso, E.J. Benjamin, M.S. Bittencourt, C.W. Callaway, A.P. Carson, A.M. Chamberlain, . . . . and C.W. Tsao (2020). Heart disease and stroke statistics - 2020 update: A Report From the American Heart Association. Circulation 141:e139-e596.
Vyas, S., D. Varshney, P. Sharma, R. Juyal, V. Nautiyal, and V. Shrotriya (2015). An overview of the predictors of symptomatic urinary tract infection among nursing students. Ann. Med. Health Sci. Res. 5:54-58.
Watso, J.C., and W.B. Farquhar (2019). Hydration status and cardiovascular function. Nutrients 11:1866.
Weiner, D.E., M.D. McClean, J.S. Kaufman, and D.R. Brooks (2013). The Central American epidemic of CKD. Clin. J. Am. Soc. Nephrol. 8:504-511.
Whitton, P.D., L.M. Rodrigues, and D.A. Hems (1978). Stimulation by vasopressin, angiotensin and oxytocin of gluconeogenesis in hepatocyte suspensions. Biochem. J. 176:893-898.
WHO (2021). Cardiovascular diseases (CVDs).
Wiitbrodt, M., and K. Barnes (2020). Hydration and cognition in the general population. Sports Science Exchange #209.
Wittbrodt, M.T., and M. Millard-Stafford (2018). Dehydration impairs cognitive performance: A meta-analysis. Med. Sci. Sports Exerc. 50:2360-2368.
Xu, C., C. Zhang, X.L. Wang, T.Z. Liu, X.T. Zeng, S. Li, and X.W. Duan (2015). Self-fluid management in prevention of kidney stones: A prisma-compliant systematic review and dose-response meta-analysis of observational studies. Medicine 94:e1042.
Yurtdas, G., N. Acar-Tek, G. Akbulut, O. Cemali, N. Arslan, A. Beyaz Coskun, and F.H. Zengin (2020). Risk factors for constipation in adults: A cross-sectional study. J. Am. Coll. Nutr. 39:713-719.
Ziegenhagen, D.J., G. Tewinkel, W. Kruis, and F. Herrmann (1991). Adding more fluid to wheat bran has no significant effects on intestinal functions of healthy subjects. J. Clin. Gastroenterol. 13:525-530.








