CONTINUOUS GLUCOSE MONITORING USE IN ATHLETES WITHOUT DIABETES
Published
May 2025
Author
Michael C. Riddell, PhD, Kristina Skroce, MSc,2 Lauren V. Turner, MSc,1 Andrea Zignoli, PhD,3 Howard C. Zisser, MD,4 J. Matthew Hinkley, PhD5

KEY POINTS
- Continuous glucose monitoring (CGM) can be used by athletes without diabetes as a novel wearable technology that provides valuable insights about individual responses to dietary choices, training, performance and recovery.
- Glucose responses in healthy athletes can be influenced by factors including meal glycemic load or composition; type, duration and intensity of exercise; stress; and other extrinsic and intrinsic factors.
- Circulating glucose levels following different meals and exercise vary considerably among athletes depending on their sport, training strategies and unique physiology.
- Endurance athletes are a sub-population of athletes whose unique glucose profiles and responses to exercise and diet continue to challenge our understanding of glucose regulation.
- As glucose regulation is influenced by numerous factors, incorporating additional training data and context may help improve CGM interpretation in the context of athletic performance.
INTRODUCTION
Wearable technology in athletics is already well-established, with competitive and recreational athletes often using various devices such as multisport smart watches, wrist- or finger-worn fitness and sleep trackers and even hydration sensors to help optimize training, competition and recovery. Recently, minimally invasive subcutaneously placed continuous glucose monitors (CGMs) have gained popularity among individuals not diagnosed with diabetes, with some high-profile elite level athletes using the technology to help optimize training and recovery (Reuters, 2024). While the potential of CGMs in the field of sports science and elite sports is unclear, some professional athletes and teams are currently using this technology to help guide nutritional strategies and guard against overtraining. While a previous Sports Science Exchange (SSE) article featured information on CGM use for physically active individuals with type 1 diabetes (Riddell et al., 2024), this article highlights the current state of knowledge of CGM use by athletes not living with diabetes, and identifies various research gaps related to the use of CGMs in this population for training, competition and recovery.
CONTINUOUS GLUCOSE MONITOR OVERVIEW
CGMs were first developed in the late 1990s as professional tools for physicians to assess glucose management and protect against hypoglycemia in patients living with type 1 diabetes who require daily insulin administration for survival (Riddell et al., 2024). Personal CGMs then became more widely used by patients with type 1 or type 2 diabetes to assess how foods, exercise and glucose lowering drugs impacted their own glycemia. Early CGM systems were hindered by bulky setups, short wear times, frequent finger-stick calibrations and poor accuracy. In contrast, modern CGMs are much smaller (about the size of a coin), factory-calibrated, more accurate and more affordable, costing less than $10 USD/day (Didyuk et al., 2021). Some CGM systems are now commercially available online or in pharmacies and can be purchased without a prescription.
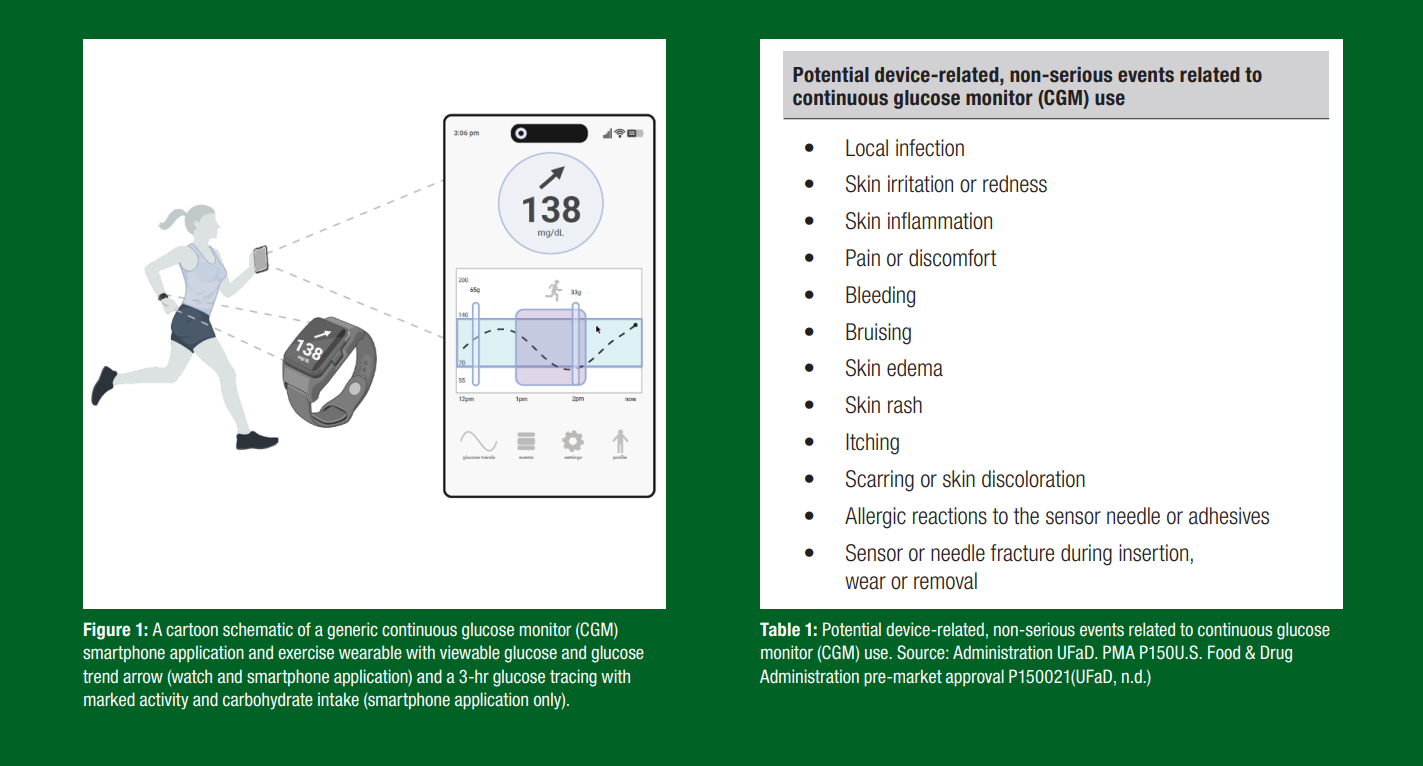
A typical CGM device consists of a disposable sensor that lasts 7–14 days, with a small filament (<0.4 mm) inserted ~5 mm into the subcutaneous fluid. This sensor is commonly placed on the upper arm, abdomen or lower back, with evidence suggesting that arm placement may provide more accurate glucose readings during exercise (Coates et al., 2023). The sensor typically has an integrated Bluetooth® (Diedisheim et al., 2023). Data are typically available with a 1–15 min sampling period with cloud-based analytics and mobile application integration (e.g., LibreLink, Dexcom Stelo).
Technically, CGMs measure glucose concentration in the interstitial fluid rather than directly in the blood. This is an important difference since interstitial glucose levels often lag behind blood glucose levels, particularly during rapid changes like post-meal “spikes” or exercise drops/rises (Scuffi & Italy, 2010). First day sensor accuracy typically suffers, and prolonged use of a CGM in the same location can also trigger a local immune response, reducing sensor accuracy over time (Joseph et al., 2018). In most current CGMs, sensor accuracy is typically robust from days 2-10, with <10% mean absolute relative difference (MARD) from laboratory-assessed plasma glucose concentration in persons with diabetes (Oliver et al., 2024) . Accuracy is deemed to be acceptable, but perhaps a little worse (MARD <15%), in persons without diabetes who are exercising (Skroce et al., 2025). Accuracy may also be affected in some CGMs by certain medications (e.g., acetaminophen, hydroxyurea, etc.), high levels of micronutrients such as vitamin C, hydration status and compression to the local circulation, which can result in false “compression lows” (Bellido et al., 2023).
While generally safe (see Table 1 for possible risks/adverse events) (Diedisheim et al., 2023), precautions should be taken in specific situations, such as removing the sensor before magnetic resonance imaging (MRI) or diathermy treatments and seeking medical attention if the sensor filament (wire) breaks under the skin.
A typical CGM device shows considerable data to the user including current glucose concentration, glucose rates of change and historical values (Figure 1). CGMs generate a significant amount of data, >4,000 glucose measurements during a 14-d wear period (5 min sampling time). Key CGM metrics provided in some apps or dashboards also include 24-hr average glucose, time spent in target glucose zones and daily glucose variability. Understanding this data, particularly for athletes and its impact on performance, requires guidance. For instance, one important CGM metric is the coefficient of variation (CV), which expresses glucose fluctuations around a central value. For people with diabetes, a CV below 36% is often recommended for optimal glucose control (Martinez et al., 2021). However, what is considered “healthy”, “suboptimal” or “optimal” for athletes without diabetes remains unclear (Flockhart & Larsen, 2024). In fact, many healthy elite-level athletes who do not have diabetes appear to have significant glucose excursions from what is thought to represent the so-called “normal glycemic range”, often with glucose levels well above 140 mg/dL (>7.8 mmol/L) or below 70 mg/dL (<3.9 mmol/L) (Skroce et al., 2025). The next sections explore how these glucose fluctuations may influence athletic performance and what CGM values athletes may wish to strive to achieve.
WHAT IS A 'NORMAL' CGM READING IN AN ATHLETE WITHOUT DIABETES?
In individuals without diabetes, interstitial glucose levels are typically between ~70-140 mg/dL (3.9-7.8 mmol/L) (Shah et al., 2019). In a large cohort of healthy adults, the mean and standard deviation 24-hr glucose was found to be 99 ± 7 mg/dL (5.5 ± 0.4 mmol/L), and the mean within-individual CV was 17 ± 3%. The median percentage of time spent between 70 and 140 mg/dL was 96% (interquartile range was 93-98) (Shah et al., 2019). CGM values are near identical in healthy non-diabetic youth according to another smaller study (DuBose et al., 2022) .
However, it is important to recognize that athletes may experience more glucose variability due to differing nutritional strategies and/or intense exercise (Skroce et al., 2025). Elite athletes may spend ~10-20% of their waking day with glucose levels >140 mg/dL (>7.8 mmol/L), and ~5-7% of the time with glucose levels <70 mg/dL (<3.9 mmol/L) (Dela et al., 1991; Flockhart et al., 2021; Phielix et al., 2019). Based on our knowledge and experiences with a large cohort database (Skroce et al., 2024a), we often see large glucose rises in elite level endurance athletes (cyclists, marathon runners, etc.) during competition, with rather low levels at other points in the day (pre feeding, sleep, etc.). For example, recent evidence suggests that elite-level male soccer players (i.e., European Professional Footballers) spend considerable time with glucose levels >140 mg/dL (>7.8 mmol/L), and much of their time in a hyperglycemic range (>180 mg/dL, >10 mmol/L) during intense match play (Skroce et al., 2024b). In another study of female Union
Cycliste Internationale (UCI), world tour cyclists using CGMs profiled over a 9-d cycling training camp, mean glucose levels were 108±9 mg/dL (6.0±0.5 mmol/L) during exercise, but the mean maximum and minimum in-ride glucose levels were 144±14 mg/dL (8.0±0.8 mmol/L) and 74±10 mg/dL (4.1±0.56 mmol/L), respectively (Hamilton et al., 2024). No associations between in-ride or overnight glycemia and cycling performance in the female UCI cyclists were found. Slightly elevated CGM values were also reported by Ishihara et al. (2020) in a group of elite male and female runners during a 160 km ultramarathon event (134±19 mg/dl, 7.4±1.1 mmol/L).
While the ideal glucose concentration for competition is not yet known for athletes, CGMs may be useful for helping to avoid the possible deleterious effects of hypoglycemia (glucose <70 mg/dL, <3.9 mmol/L) during and after exercise by initiating carbohydrate (CHO) feeding. In the early 1920s, seminal work by Levine and colleagues (1924) examined blood glucose levels measured in athletes at the end of the Boston Marathon. Their work showed that athletes provided with CHO had elevated glucose levels and improved well-being and performance compared to the previous year, when no CHO feeding was given, and athletes experienced moderate to severe hypoglycemia at the finish of the race (venous glucose <50 mg/dL, <2.8 mmol/L, clinical symptoms similar to insulin “shock”). Building on these early findings, the recent advent of CGM use by numerous athletes without diabetes has led to the publication of several case-studies on fueling and athletic performance (Doering et al., 2019; Francois et al., 2018; Ishihara et al., 2020; Thomas et al., 2016). For instance, during one ultra-race, athletes who consumed fewer CHO had lower mean glucose levels and poorer performance (Ishihara et al., 2020). Another study of healthy athletes found that, compared to a placebo, CHO intake was associated with elevated mean glucose levels (CHO: 106±18 vs. Placebo: 88±10 mg/dL; 5.9±1.0 vs. 4.9±0.6 mmol/L) and increased time to fatigue (CHO: 113±69 vs. Placebo: 81±49 min; 6.3±3.8 vs. 4.5±2.7 mmol/L) (Elghobashy et al., 2024).
While a mean blood glucose concentration is often reported in sports medicine or exercise physiology literature, mean glucose values only offer a ‘snapshot’ of an average value at one (or more) points in time and thus fail to highlight the occurrence of individualized glucose fluctuations that can be profiled more dynamically with CGMs. Published data have now shown that sub-elite athletes spend upwards of 70% of their time with glucose levels above 106 mg/dL (6.0 mmol/L) during training and endurance events (Thomas et al., 2016). Even with high daily CHO intake (~8.5 g CHO/kg/d) to support high rates of CHO oxidation, CGM values of elite level racewalkers are in the normal glycemic range >90% time over a 24-hr period, and with typical markers of glucose variability observed (Bowler et al., 2024). However, another study demonstrated that elite athletes experienced elevated time outside the so-called “normal” glycemic range of 70-140 mg/dL (3.9-7.8 mmol/L), with episodes both above and below this “normal range" over a day compared to healthy controls (Flockhart et al., 2021). Importantly, even with these glucose fluctuations, elite and masters’ athletes, compared to the general population, have shown no long-term health effects, maintaining lower fasting glucose levels and heightened whole body insulin sensitivity (Climstein et al., 2022) and protection from various cardio-metabolic diseases (Ruiz et al., 2014). Thus, the advent of CGM has highlighted that glucose fluctuations outside the 70- 140 mg/dL glycemic range may not be disadvantageous. Frustratingly, the ideal CGM glucose range for healthy athletes is currently unclear (Flockhart & Larsen, 2024).
Based on data from >7500 healthy “athletic” CGM users (Abbott Libre Biosensor), we have observed that ~80% of all CGM values are between 70-140 mg/dL (3.9-7.8 mmol/L), with ~17%>140 mg/dL and ~3%<70 mg/dL (unpublished data) (Figure 2).
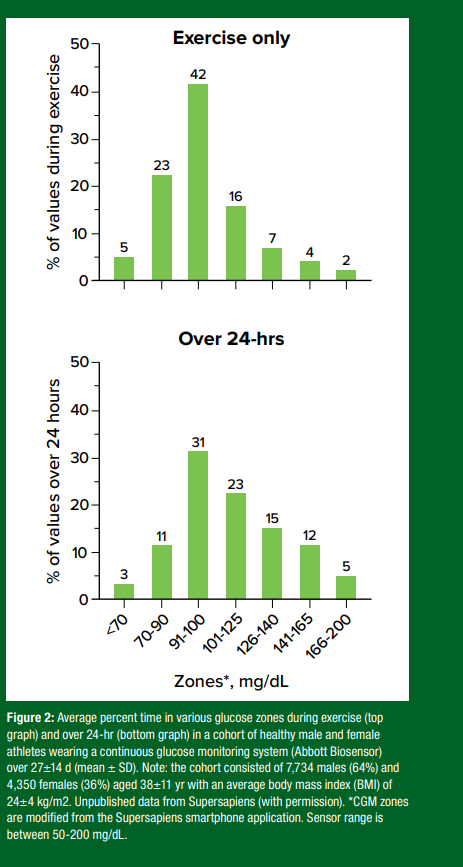
CGM values during real-world endurance exercise are also typically between 70-140 mg/dL (3.9-7.8 mmol/L) but with slightly more time below 70 mg/dL (Shah et al., 2019). If the exercise is fasted and done in a laboratory setting for a limited duration (up to ~30 min), glucose levels are typically between 80-110 mg/dL (4.4-6.1 mmol/L), regardless of the exercise intensity (Skroce et al., 2025). Considerable interindividual variation and a wide range of glucose responses within any 24-hr period have also been observed, but with glucose values typically higher during the day with meals and exercise and then falling to a nadir overnight (unpublished data). Understanding individualized glucose trends and their effects on performance is likely important, particularly when contrasting the individual glucose response to a given exercise event, such as a competitive cycling race.
POSSIBLE APPLICATIONS OF CGM IN SPORT
While typical glucose ranges still need to be established (and may be highly individualized), CGM data may be able to provide important insight on diet, fueling strategies and recovery in healthy athletes. The following sections describe how an athlete or practitioner/coach may use the CGM data to help optimize athletic performance.
CGMs and Personalized Nutrition: Individual Response to Various Food Items
CGM systems provide athletes with almost immediate feedback on glycemic responses to their nutrition choices. Glucose values, trend arrows and line graphs viewed on the CGM receiver allow users to understand their individual response to various food groups. As the popularity of CGM use increases amongst users without diabetes, so does the knowledge around intra-variability of glycemic responses.
Athletes using CGM for the first time may notice unexpected glycemic responses to certain foods, or observe inter-variability when comparing their data to other athletes.
Emerging evidence suggests that 24-hr CGM profiles are highly individualized, even for those without diabetes (Flockhart & Larsen, 2024). For example, a study of 1,002 adults without diabetes demonstrated large inter-individual variability in post-prandial responses of blood glucose, triglycerides and insulin levels between subjects following standardized meals (Berry et al., 2020). Moreover, another study examined post-prandial glucose patterns and identified three distinct “glucotypes” of increasing variability (low, moderate and severe) following an identical nutrient challenge, suggesting interpersonal variation in post-prandial glucose metabolism in people without diabetes (Hall et al., 2018).
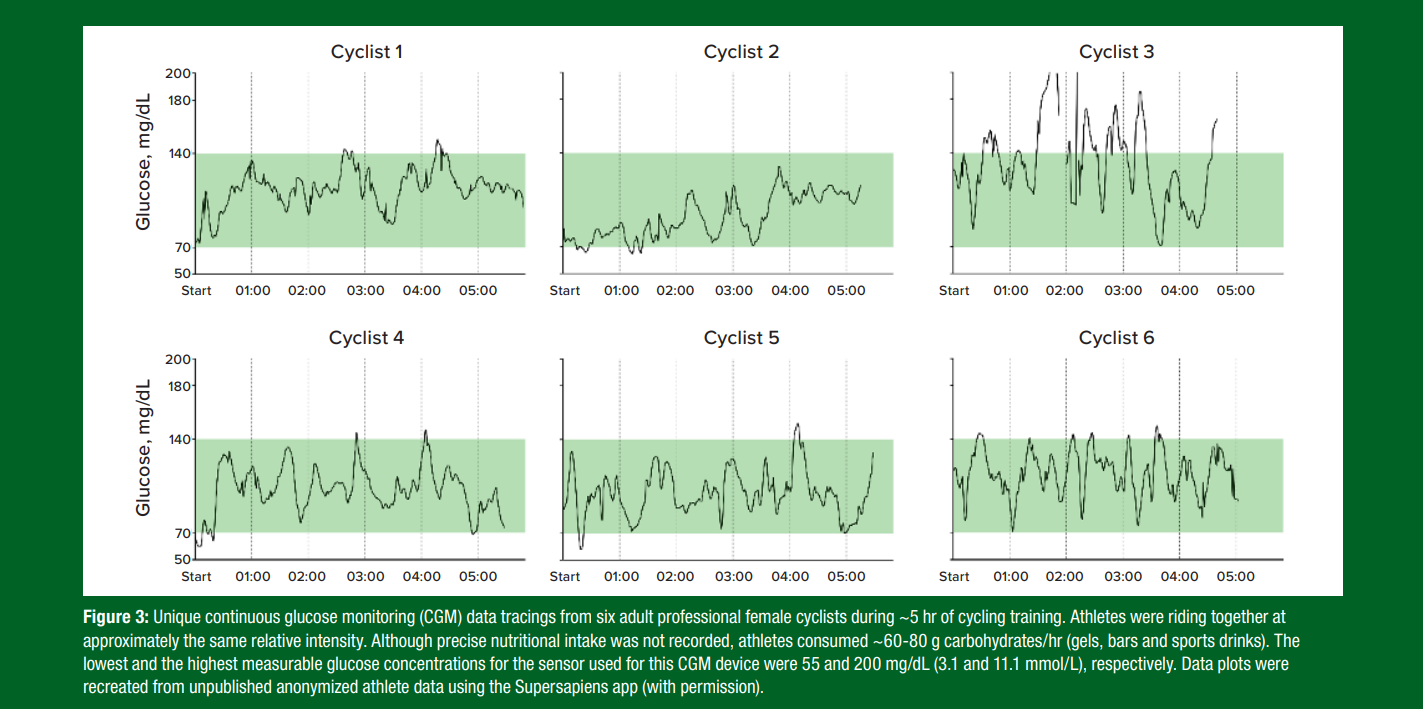
While still learning about how an athlete’s glucose profiles are influenced by diet, training and sleep patterns, CGM-guided personalized nutrition offers the potential to tailor food consumption to an individual's unique metabolic requirements and glycemic responses (Jarvis et al., 2023; Ordovas et al., 2018). For instance, observations on a team of healthy elite female cyclists showed that, despite completing the same training session at approximately the same relative intensity and similar durations, each athlete’s glucose profile was unique with some having considerable changes in their glycemia during the training sessions (Figure 3). These findings suggest that one-size-fits-all dietary guidelines for healthy eating may not adequately meet an athlete’s fueling needs to maintain glucose within a targeted range that may facilitate performance (e.g., 100-150 mg/dL, 5.6-8.3 mmol/L).
Recently, the application of machine learning technologies to personalize nutrition has been enhanced with the use of CGMs in people without diabetes (Kim et al., 2019; Tily et al., 2022). These technologies have highlighted that CHO is the main driver for increasing glucose area under the curve (AUC, a measure of glucose rise over a specified period compared to baseline), while proteins and fat can act together to favorably reduce the AUC. Moreover, a recent machine-learning model can be used to predict the glycemic response to a meal based on individual characteristics (gender, age and BMI), macronutrients and 24-hr CGM trace preceding the meal (Zignoli et al., 2024). These findings suggest that CGM has the potential for use as an educational learning tool for individuals with and without diabetes to understand how different meal compositions and exercise events can impact circulating glucose levels.
How to Use CGM Pre-exercise for Selecting and Implementing Nutritional Strategies that Maintain Glycemia in Targeted Zone
By experimenting with CGM data and various nutritional strategies, athletes can learn more about their unique glycemic profiles to various foods, exercise training and competition events. This may allow athletes to determine their optimal pre-exercise, “in-exercise” and post-exercise fueling strategies to optimize performance. Using nutrient logs alongside CGM might further assist athletes in determining the ideal timing, amount and type of CHO to be consumed to help sustain performance (Jeukendrup & Killer, 2011; Rothschild et al., 2020). CGM can also help detect and prevent excessive glucose fluctuations after pre-exercise CHO consumption, particularly at exercise onset.
Mitigating Reactive Hypoglycemia: Reactive hypoglycemia occurs when the combination of increased insulin secretion driven by food ingestion and insulin-independent glucose uptake driven by exercise result in a fall of blood glucose below the hypoglycemic threshold (<70 mg/dL, <3.9 mmol/L) (Jeukendrup & Killer, 2011) (Figure 3, cyclists 2, 4 and 5). This transient event, often observed within the first 30 min of exercise, is sometimes associated with negative symptoms such as weakness, nausea and dizziness (Foster et al., 1979). A recent study of 6,761 users without diabetes reported that reactive hypoglycemia was detected in ~8% of users, with the majority of events when pre-exercise food timing was ~30-90 min before exercise (Zignoli et al., 2023). These results suggest that pre-exercise food timing may increase the risk of unfavorable reactive hypoglycemia in some athletes, but CGM could offer an opportunity to better observe and manage this risk.
Rebound Hyperglycemia Mitigation: CGM might help identify athletes susceptible to rebound hyperglycemia, a condition where an excessive insulin response to pre-exercise carbohydrate intake (especially high- glycemic index (GI) foods) initially lowers glucose levels, triggering an excessive release of counter-regulatory hormones (such as glucagon) which subsequently raise glucose to hyperglycemic levels (>140 mg/ dL, >7.8 mmol/L) (Figure 3, cyclist 3). CGM can offer athletes the opportunity to observe these patterns and refine and improve their pre-exercise nutritional strategies. This could include choosing low GI carbohydrates, ingesting carbohydrate just before exercise or during a warm-up or avoiding carbohydrates in the 90 min before exercise (Jeukendrup & Killer, 2011).
How Athletes can use CGM Data During Prolonged Exercise to Help Avoid In-exercise Hypoglycemia or Hyperglycemia
Prolonged endurance exercise, such as marathon running, can result in significant decreases in glucose by the end of an event, placing the athlete in hypoglycemia which can significantly impair cognitive function and endurance performance (Levine et al., 1924). Strategic carbohydrate feeding during exercise can help to mitigate hypoglycemia, delay fatigue and enhance performance (Coyle & Coggan, 1984). However, the optimal timing and dose of carbohydrate ingestion during exercise is likely highly individualized (Kerksick et al., 2017).
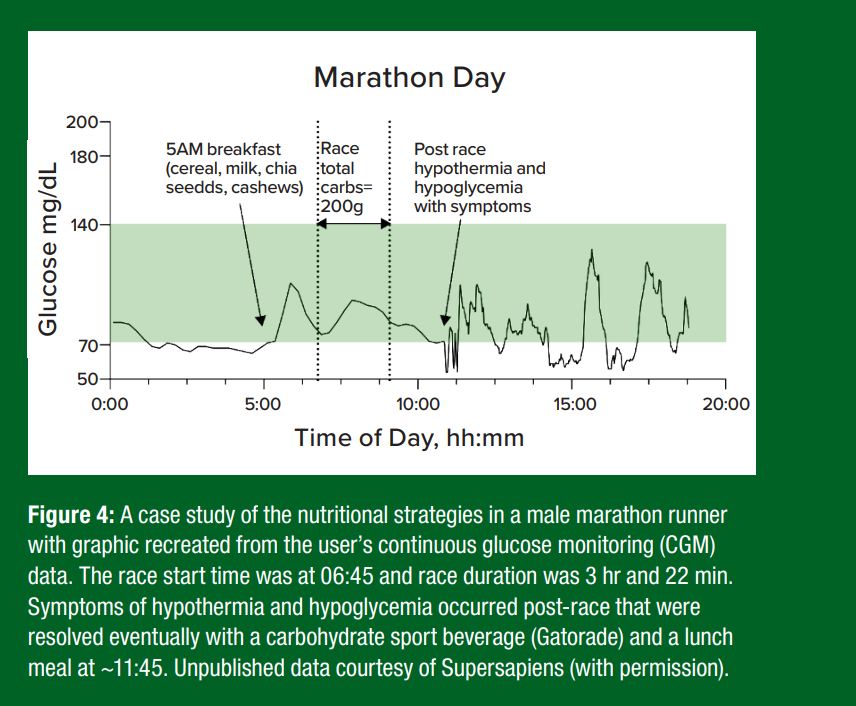
As shown in a case study of a male marathoner (Figure 4), the pre- race ingestion of high-glycemic index (GI) foods, such as commercial North American cereals with milk can cause glucose to spike and then drop pre-race. At the race start and throughout the race, the frequent consumption of high GI and digestible CHO (e.g., gels, sport drinks) can help maintain glycemia in a target range.
CGM offers a practical tool for athletes to fine tune their strategies to help prevent hypoglycemia and potentially improve endurance performance in training and competition. For instance, a recent study reported that athletes participating in a single-day 160-km ultra-trail race who consumed less CHO throughout the race tended to have lower glucose levels and took longer to complete the race compared to those consuming more CHO (Ishihara et al., 2020). Additionally, in a 5-d adventure race, athletes experienced brief periods of hypoglycemia and increased glycemic variability on race days compared to non-race days (Francois et al., 2018). Para cyclists (hand bikers, tandem cyclists, track and road-race cyclists) have typical 24-hr mean glucose levels, but some mild hyperglycemia during exercise (Weijer et al., 2024). These findings suggest that CGM can provide valuable insights into an athlete’s glucose trends, helping to minimize glycemic fluctuations and optimize performance during prolonged exercise.
Using CGM to Observe the Impact of Training Load and Overtraining on Overnight Glucose and Performance
Heavy exercise, a race day or sub-optimally fueling may impact overnight glucose levels, which can also be evaluated retrospectively with CGM. Normally, overnight CGM traces follow a U-shaped curve, with the glucose nadir occurring before waking up at ~4 AM for most healthy physically active individuals (Bowler et al., 2024; Flockhart et al., 2021; Skroce et al., 2024a). The overnight glucose nadir appears to be slightly lower (~4.5 mg/dL, 0.25 mmol/L) and shifted to later in the morning (~6 AM) in elite-level endurance athletes (Bowler et al., 2024). Moreover, episodes of nocturnal hypoglycemia (<47 mg/dL,
<4.0 mmol/L) were also observed between 3 and 7 AM in elite-level endurance athletes, a phenomenon not typically present in endurance athletes training less than 7 hr/week (Bowler et al., 2024; Flockhart et al., 2021). Mild nocturnal hypoglycemia was also observed in the para-athlete cyclists and in particular the athletes with spinal cord injury (Weijer et al., 2024), which suggests some impairment in counter- regulation to hypoglycemia likely due to autonomic dysfunction (Rickels, 2019). A small observational study of professional road cyclists found slightly elevated CGM values during daily training (114±9.7 mg/ dL, 6.4±0.54 mmol/L) and overnight euglycemia (95±9.4 mg/dL, 5.3±0.52 mmol/L), thereby suggesting that they were matching fueling needs well (Hamilton et al., 2024). Thus, overnight glycemia appears to be impacted significantly by recent training events and post activity nutrient intake.
In addition to overnight glucose monitoring, CGM can be used as a tool to detect overreaching (i.e., poor recovery between training sessions which can increase muscle soreness, and lower performance) and/or overtraining (i.e., continuing to train despite overreaching which can lead to long term decrements in performance). Lower fasting blood glucose levels have been proposed as an indicator of overreaching in elite athletes (Ishigaki et al., 2005), but this phenomenon has only recently been examined using CGM in a handful of studies. Some evidence suggests that failure to increase circulating glucose levels during high-intensity exercise could be indicative of overreaching or possibly a maladaptation to the training load, potentially due to a reduced catecholamine response during exercise (Flockhart et al., 2021). More specifically, recreationally active individuals who underwent a 3-week high-intensity interval training intervention showed reduced exercising capillary glucose concentrations during high-intensity cycling during a performance plateau, which resolved after recovery (Flockhart et al., 2021; 2022). This performance plateau was accompanied by decreased mitochondrial respiration and increased fat utilization during submaximal exercise, though no changes were observed in muscle glycogen stores, fasting free fatty acids or resting metabolic rate, ruling out insufficient energy availability as the cause of lower circulating glucose concentrations (Flockhart et al., 2021; Koehler et al., 2016). If circulating glucose and/or glycogen utilization during exercise are indeed altered with overreaching in endurance-trained athletes, CGM could serve as an additional tool to monitor training load and potentially detect signs of overtraining (Bowler et al., 2023; Coates et al., 2024). Considering the high CHO intake demands of multi-day exercise that can be 7-10 g/kg/d for endurance athletes (Burke et al., 2001), lower CGM values overnight or during an activity might be a sign of under fueling and/or inadequate glycogen replenishment.
CGM and Competition Stress
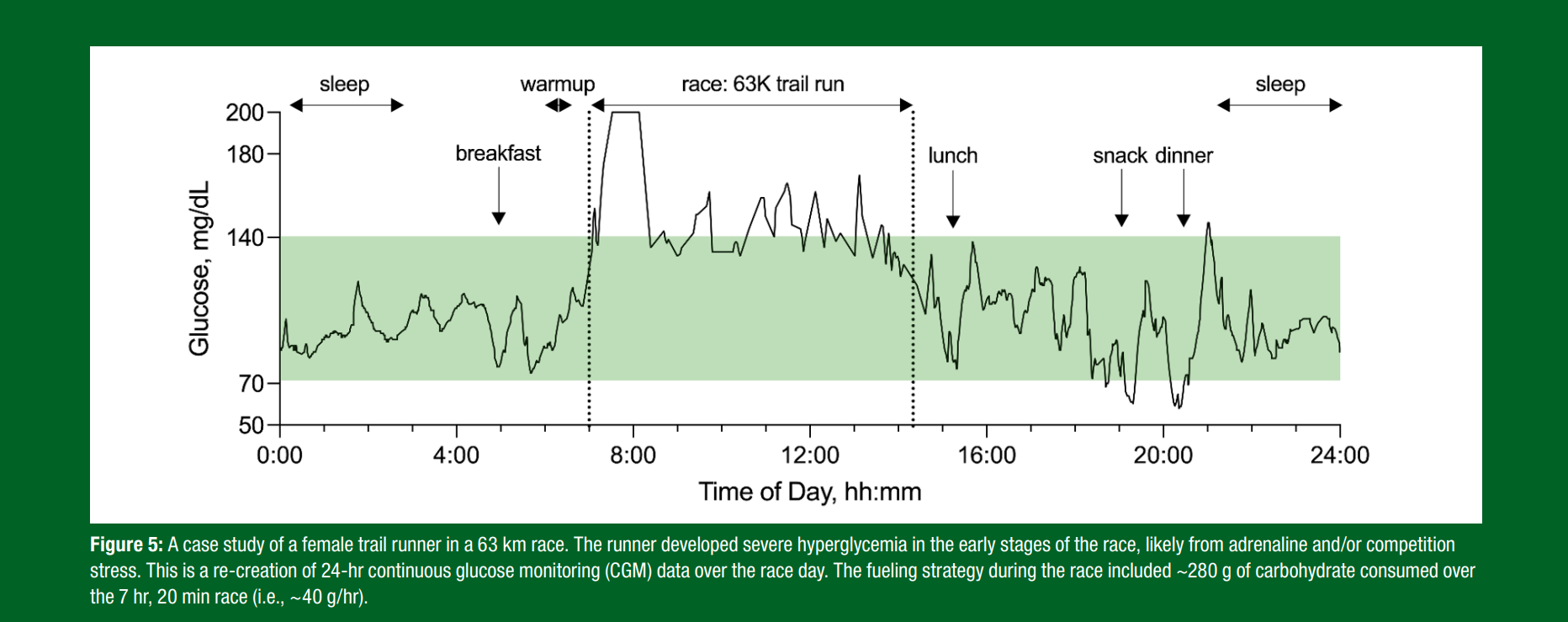
Emerging evidence has shown that competitive events and races increase glycemia relative to training and/or non-competitive events. It was recently shown that elite European footballers tend to have a rise in CGM values during the first half of a game, but then a drop by the end of the second half. In another example, shown in Figure 5, competition stress resulted in early race hyperglycemia that was resolved 30 min after the race began. While research is limited, there is no evidence that the rise in glycemia reduces athletic performance. Many of the highest-level athletes appear to have elevations in glycemia at the onset of a competitive event such as a marathon, half-marathon, team cycling event and/or team sport (Skroce et al., 2025). The rise in glycemia, however, is usually temporary and as such, fuel provision is still required for performance reasons, although more research is required in this area. Currently, while other measures may be better assessment tools (i.e., heart rate variability, galvanic skin response, salivary cortisol and/or questionnaires), more research is needed to determine whether fluctuations in glucose levels are impacted by competition stress.
POTENTIAL RISKS OF USING CGM
While top misconceptions of CGM use in athletes are outlined below, the largest concern surrounding CGM use in athletes is the potential for developing disordered eating behaviors. Excessive glucose data exposure might induce a type of "glucorexia," where athletes become overly sensitive to minor, non-relevant and normal fluctuations in their glucose levels (Bowler et al., 2023). This heightened awareness of CGM readings may encourage obsessive behaviours and anxiety around nutrition and performance, even in athletes without a history of psychological disorders.
Top Misconceptions of CGM in Healthy Populations
- All glucose spikes and variability are bad
- Glucose levels should always be analyzed in the context of the broader picture. Not all glucose spikes are bad because not all spikes are the same. For example, a spike induced by high-intensity exercise is a “good” spike, while frequent poor nutritional choices can lead to a series of “bad” spikes and potentially negative health consequences.
- Glucose is continuously stable in healthy athletes
- Individual glucose responses to foods, training and stress will lead to fluctuations of glucose levels which are “normal” and physiological.
- CGMs are less accurate than blood glucose meters
- Comparing blood glucose meter and CGM readings when glucose is changing quickly can be misleading, since blood glucose changes before interstitial fluid glucose.
- Glucose value is more important than the trend itself
- The dynamics and trends of glucose are of more significance and relevance than the actual glucose value for people without diabetes.
- CGM is a proxy for fuel (fueling sensor)
- CGM cannot measure glycogen in the muscle. The CGM value is showing the absorption of glucose from the liver and intestine, but it does not give information about the muscle glycogen content, a primary fuel gauge for athletic performance (Riddell et al., 2024).
- Higher CGM leads to better performance
- Although athletes anecdotally report better performances when glucose levels were “higher” than their “usual” trends, there is still lack of scientific evidence to support this claim. It is important to underline that each person has a highly individual glucose response to meals, training and/or competition and stress
CONCLUSIONS
CGM is emerging as a valuable tool for athletes to better gauge and monitor their individualized glucose responses to nutrition, training and competition. While the use of CGM to optimize circulating glucose levels is increasingly recognized as beneficial for enhancing athletic performance and health, glucose regulation is a complex process influenced by several factors including nutritional intake, training status, exercise intensity and duration and/or metabolic fitness. Although most daily blood glucose values fall within the euglycemic range of 70-140 mg/dL (3.9-7.8 mmol/L), research using CGM has shown that healthy athletes can experience significant, and sometimes unpredictable, fluctuations in glycemia, including brief periods of both hypo- and hyperglycemia. The use of CGM can be an important tool for athletes to better understand their unique glycemic patterns to various foods and nutrients, the timing of nutrient intake relative to exercise events, their unique glycemic response to competition and their fuel recovery needs.
Over the last 12 months, MCR has served on scientific advisor boards for embecta, Dexcom Inc, Eli Lilly, Indigo Diabetes, Insulet, Novo Nordisk, Spiden AG, Supersapiens, Zealand Pharma and Zucara Therapeutics. MCR has also provided Continuing Medical Education lectures for Dexcom, Novo Nordisk, Sanofi, Roche Diabetes Care and Insulet. MCR’s research has been supported with funding (or in-kind contributions) from Dexcom, Eli Lilly, Supersapiens and Zucara Theraputics. MCR holds stock and/or commercial interest in Supersapiens and Zucara Therapeutics. KS, AZ and HCZ were employed or consultants for Supersapiens (TT1 Products, INNC, Atlanta, GA, USA) until 1st of March 2024. Supersapiens provided sensors, but no funding for KS's research. JMH is employed by the Gatorade Sports Science Institute, a division of PepsiCo R&D. The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc. All other authors have no disclosures to declare.
REFERENCES
Bellido, V., G. Freckman, A, Pérez, and R.J. Galindo (2023). Accuracy and potential interferences of continuous glucose monitoring sensors in the hospital. Endoc. Prac. 29:919–927.
Berry, S.E., A.M. Valdes, D.A. Drew, F. Asnicar, M. Mazidi, J. Wolf, J. Capdevila, G. Hadjigeorgiou, R. Davies, H.A. Khatib, C. Bonnett, S. Ganesh, E. Bakker, D. Hart, M. Mangino, J. Merino, I. Linenberg, P. Wyatt, J.M. Ordovas, C.D. Gardner, L.M. Delahanty,
A.T. Chan, N. Segata, P.W. Franks, and T.D. Spector (2020). Human postprandial responses to food and potential for precision nutrition. Nat. Med. 26:964–973.
Bowler, A.-L.M., J. Whitfield, L. Marshall, V.G. Coffey, L.M. Burke, and G.R. Cox (2023). The use of continuous glucose monitors in sport: Possible applications and considerations. Int. J. Sport Nutr. Exerc. Metab. 33:121–132.
Bowler, A.-L.M., L.M. Burke, V.G. Coffey, and G.R. Cox (2024). Day-to-day glycemic variability using continuous glucose monitors in endurance athletes. J. Diab. Sci. Technol. Online ahead of print. PMID:38726672
Burke, L.M., G.R. Cox, N.K. Cummings, and B. Desbrow (2001). Guidelines for daily carbohydrate intake. Sports Med. 31:267–299.
Climstein, M., J. Walsh, K. Adams, T. Sevene, T. Heazlewood, and M. DeBeliso (2022).
Prevalence of hyperglycemia in masters athletes. PeerJ, 10:e13389.
Coates, A.M., J.N. Cohen, and J.F. Burr (2023). Investigating sensor location on the effectiveness of continuous glucose monitoring during exercise in a non-diabetic population. Eur. J. Sport Sci. 23:2109–2117.
Coates, A.M., K.M.A. Thompson, M.M. Grigore, R.E. Baker, C. Pignanelli, A.A. Robertson,
S.M. Frangos, C.P. Cheung, and J.F. Burr (2024). Altered carbohydrate oxidation during exercise in overreached endurance athletes is applicable to training monitoring with continuous glucose monitors. Scand. J. Med. Sci. Sports 34:e14551.
Coyle, E.F. and A.R. Coggan (1984). Effectiveness of carbohydrate feeding in delaying fatigue during prolonged exercise. Sports Med. 1:446–458.
Dela, F., K.J. Mikines, M.V. Linstow, and H. Galbo (1991). Twenty-four-hour profile of plasma glucose and glucoregulatory hormones during normal living conditions in trained and untrained men. J. Clin. Endocrinol. Metab. 73:982–989.
Didyuk, O., N. Econom, A. Guardia, K. Livingston, and U. Klueh (2021). Continuous glucose monitoring devices: Past, present, and future focus on the history and evolution of technological innovation. J. Diab. Sci. Technol. 15:676–683.
Diedisheim, M., C. Pecquet, J.-B. Julla, A. Carlier, L. Potier, A. Hartemann, S. Jacqueminet,
T. Vidal-Trecan, J.-F. Gautier, D.D. Laforgue, G. Fagherazzi, R. Roussel, E. Larger, A. Sola-Gazagnes, and J.-P. Riveline (2023). Prevalence and description of the skin reactions associated with adhesives in diabetes technology devices in an adult population: Results of the CUTADIAB study. Diab. Technol. Ther. 25:279–286.
Doering, T.M., G.R. Cox, J.L. Areta, and V.G. Coffey (2019). Repeated muscle glycogen supercompensation with four days’ recovery between exhaustive exercise. J. Sci. Med. Sport 22:907–911.
DuBose, S.N., L.G. Kanapka, B. Bradfield, M. Sooy, R.W. Beck, and A.K. Steck (2022). Continuous glucose monitoring profiles in healthy, nondiabetic young children. J. Endocrine Soc. 6:1-7.
Elghobashy, M.E., A.J. Richards, R. Malekzadeh, D. Patel, L.V. Turner, J.F. Burr, G.A. Power,
R. Laham, M.C. Riddell, and A.J. Cheng (2024). Carbohydrate ingestion increases interstitial glucose and mitigates neuromuscular fatigue during single-leg knee extensions. Med. Sci. Sports Exerc. 56:1495–1504.
Flockhart, M., and F.J. Larsen (2024). Continuous glucose monitoring in endurance athletes: Interpretation and relevance of measurements for improving performance and health. Sports Med. 54:247–255.
Flockhart, M., L.C. Nilsson, S. Tais, B. Ekblom, W. Apró, and F.J. Larsen (2021). Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 33:957-970.
Flockhart, M., L.C. Nilsson, B. Ekblom, and F.J. Larsen (2022). A simple model for diagnosis of maladaptations to exercise training. Sports Med. Open 8:136.
Foster, C., D.L. Costill, and W.J. Fink (1979). Effects of preexercise feedings on endurance performance. Med. Sci. Sports 11:1–5.
Francois, M.E., S.D. Cosgrove, N.M. Walker, S.J. Lucas, and K.E. Black (2018). Physiological responses to a five-day adventure race: Continuous blood glucose, hemodynamics and metabolites the 2012 GODZone field-study. J. Exerc. Sci. Fit. 16:78–82.
Hall, H., D. Perelman, A. Breschi, P. Limcaoco, R. Kellogg, T. McLaughlin, and M. Snyder (2018). Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 16:e2005143.
Hamilton, R., O.M. McCarthy, S.C. Bain, and R.M. Bracken (2024). Continuous measurement of interstitial glycaemia in professional female UCI world tour cyclists undertaking a 9‐ day cycle training camp. Eur. J. Sport Sci. 24:1573–1582.
Ishigaki, T., K. Koyama, J. Tsujita, N. Tanaka, S. Hori, and Y. Oku (2005). Plasma leptin levels of elite endurance runners after heavy endurance training. J. Physiol. Anthrolpol. Appl. Human Sci. 24:573.
Ishihara, K., N. Uchiyama, S. Kizaki, E. Mori, T. Nonaka, and H. Oneda (2020). Application of continuous glucose monitoring for assessment of individual carbohydrate requirement during ultramarathon race. Nutrients 12:1121.
Jarvis, P.R.E., J.L. Cardin, P.M. Nisevich-Bede, and J.P. McCarter (2023). Continuous glucose monitoring in a healthy population: understanding the post-prandial glycemic response in individuals without diabetes mellitus. Metabolism 146:155640.
Jeukendrup, A.E. and S.C. Killer (2011). The myths surrounding pre-exercise carbohydrate feeding. Ann. Nutr. Metab. 57(Suppl 2):18–25.
Joseph, J.I., G. Eisler, D. Diaz, A. Khalf, C. Loeum, and M.C. Torjman (2018). Glucose sensing in the subcutaneous tissue: Attempting to correlate the immune response with continuous glucose monitoring accuracy. Diab. Technol. Ther. 20:321–324.
Kerksick, C.M., S. Arent, B.J. Schoenfeld, J.R. Stout, B. Campbell, C.D. Wilborn, L. Taylor, D. Kalman, A.E. Smith-Ryan, R.B. Kreider, D. Willoughby, P.J. Arciero, T.A. VanDusseldorp,
M.J. Ormsbee, R. Wildman, M. Greenwood, T.N. Ziegenfuss, A.A. Aragon, and J. Antonio (2017). International society of sports nutrition position stand: Nutrient timing.
J. Int. Soc. Sports Nutr. 14:33.
Kim, J.S., K. Nam, and S.-J. Chung (2019). Effect of nutrient composition in a mixed meal on the postprandial glycemic response in healthy people: A preliminary study. Nutr. Res. Prac. 13:126–133.
Koehler, K., N.R. Hoerner, J.C. Gibbs, C. Zinner, H. Braun, M.J. DeSouza, and W. Schaenzer (2016). Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J. Sports Sci. 34:1921– 1929.
Levine, S.A., B. Gordon, and C.L. Derick (1924). Some changes in the chemical constituents of the blood following a marathon race: with special reference to the development of hypoglycemia. J. Am. Med. Assoc. 82:1778–1779.
Martinez, M., J. Santamarina, A. Pavesi, C. Musso, and G.E. Umpierrez (2021). Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diab. Res. Care 9:e002032.
Oliver, N., M. Reddy, and L. Leelarathna (2024). Continuous glucose sensor accuracy: beyond the headline metric. Lancet Diab. Endocrinol. 12:934-938.
Ordovas, J.M., L.R. Ferguson, E.S. Tai, and J.C. Mathers (2018). Personalised nutrition and health. BMJ:361.
Phielix, E., P. Begovatz, S. Gancheva, A. Bierwagen, E. Kornips, G. Schaart, M.K.C. Hesselink,
P. Schrauwen, and M. Roden (2019). Athletes feature greater rates of muscle glucose transport and glycogen synthesis during lipid infusion. JCI Insight 4:e127928.
Reuters. (2024, 10. June). Olympic athletes turn to diabetes tech in pursuit of medals. https://www.cnn.com/2024/06/10/sport/paris-olympics-diabetes-tech-spt-intl/index.html
Rickels, M.R. (2019). Hypoglycemia‐associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann. New York Acad. Sci. 1454:68–79.
Riddell, M.C., L.V. Turner, K. Skroce, H.C. Zisser, and M. Hinkley (2024). Continuous glucose monitoring and the athlete with type 1 diabetes. SSE #256.
Rothschild, J.A., A.E. Kilding, and D.J. Plews (2020). What should I eat before exercise? Pre-exercise nutrition and the response to endurance exercise: Current prospective and future directions. Nutrients 12:3473.
Ruiz, J.R., C. Fiuza-Luces, N. Garatachea, and A. Lucia (2014). Reduced mortality in former elite endurance athletes. Int. J. Sports Physiol. Perf. 9:1046–1049.
Scuffi, C. and S. Italy (2010). Interstitium versus blood equilibrium in glucose concentration and its impact on subcutaneous continuous glucose monitoring systems. Eur. Endocrinol. 10:36.
Shah, V.N., S.N. DuBose, Z. Li, R.W. Beck, A.L. Peters, R.S. Weinstock, D. Kruger, M. Tansey, D. Sparling, S. Woerner, F. Vendrame, R. Bergenstal, W.V. Tamborlane, S.E. Watson, and J. Sherr (2019). Continuous glucose monitoring profiles in healthy nondiabetic participants: A multicenter prospective study. J. Clin. Endocrinol. Metab. 104:4356–4364.
Skroce, K., A. Zignoli, F.Y. Fontana, F.M. Maturana, D. Lipman, A. Tryfonos, M.C. Riddell, and
H.C. Zisser (2024a). Real world interstitial glucose profiles of a large cohort of physically active men and women. Sensors 24:744.
Skroce, K., M. Riddell, N. Mihic, A. Zignoli, D. Lipman, and H. Zisser (2024b). Glucose monitoring profiles in professional football players without diabetes: match analysis and comparison with an active population. 60th EASD Annual Meeting, 9-13 September 2024. https://www.easd.org/media-centre/home.html#!
Skroce, K., L.V. Turner, F.Y. Fontana, S. Bettega, S., Nardelli, A. Jeukendrup, H.C. Zisser, F. Schena, C. Tarperi, and M.C. Riddell (2025). Assessing the accuracy of a continuous glucose monitoring system across varying exercise intensities and blood lactate concentrations in healthy male athletes. J. Diab. Sci. Technol. 19:274-276.
Thomas, F., C.G. Pretty, T. Desaive, and J.G. Chase (2016). Blood glucose levels of subelite athletes during 6 days of free living. J. Diab. Sci. Technol. 10:1335–1343.
Tily, H., E. Patridge, Y. Cai, V. Gopu, S. Gline, M. Genkin, H. Lindau, A. Sjue, I. Slavov, A. Perlina, N. Klitgord, H. Messier, M. Vuyisich, and G. Banavar (2022). Gut microbiome activity contributes to prediction of individual variation in glycemic response in adults. Diab. Ther. 13:89–111.
UFaD, A. (2014). PMA P150021: FDA Summary of Safety and Effectiveness Data. Retrieved October 21, 2024, from https://www.accessdata.fda.gov/cdrh_docs/pdf15/ P150021B.pdf
Weijer, V., R. van der Werf, M. van der Haijden, A. Jeukendrup, L.J.C. van Loon, and J.-W. van Dijk (2024). Continuous glucose monitoring in para cyclists: An observational study. Eur. J. Sport Sci. 12:1809-1819.
Zignoli, A., F.Y. Fontana, D.J. Lipman, K. Skroce, F.M. Maturana, and H.C. Zisser (2023). Association between pre‐exercise food ingestion timing and reactive hypoglycemia: Insights from a large database of continuous glucose monitoring data. Eur. J. Sport Sci. 23:2340–2348.
Zignoli, A., K. Skroce, D.J. Lipman, and H.C. Zisser (2024). Personalized nutrition and machine-learning: Exploring the scope of continuous glucose monitoring in healthy individuals in uncontrolled settings. Biomed. Signal Process. Control 90:105809.








