REGULATION OF FAT METABOLISM DURING EXERCISE
Published
July 2020
Author
Lawrence L. Spriet, PhD; Rebecca K. Randell, PhD
Topics

KEY POINTS
- Fat and carbohydrate (CHO) are the main fuels for aerobic metabolism during exercise in a well-fed person.
- Fat is the dominant energy source at low aerobic power outputs (< 40% VO2max) and provides ~50% of the required energy during moderate intensity exercise (~40-65% VO2max). The contribution from fat decreases at higher power outputs as CHO becomes the main fuel.
- Fat oxidation also contributes energy during recovery from a single bout of exercise, and in the rest or low power output recovery periods between intense exercise bouts common to stop-and-go sports.
- The regulation of fat metabolism in skeletal muscle during exercise is complex and involves many sites of control. Activation of fat oxidation at the onset of exercise is slower than CHO and is designed for long-term low to moderate intensity exercise.
- The decrease in fat oxidation with intense aerobic exercise occurs at several regulatory sites inside and outside skeletal muscle.
INTRODUCTION
Fat and carbohydrate (CHO) are the primary fuels for the generation of energy during aerobic exercise in well fed individuals. The relative contribution of these pathways is determined primarily by the exercise intensity and duration of exercise, but is also affected by training status, preceding diet, sex and environmental conditions. In the aerobic exercise domain up to ~100% of maximal oxygen uptake (VO2max), CHO is the dominant fuel, as CHO-based oxidative metabolism can be activated quickly, provide all of the fuel at high aerobic power outputs (> 85-90% VO2max) and is a more efficient fuel (kcal/L O2 used) when compared to fat. However, while fat-based oxidative metabolism is slower to activate and provides less fuel as exercise intensities increase above 65-75% VO2max, it has a much larger capacity than CHO oxidation. Fat is designed to be a helper fuel during aerobic exercise and is the dominant energy source at low power outputs (< 40% VO2max) and provides large amounts of energy during moderate intensity exercise (~40-65% VO2max). If exercise at ~50-60% VO2max is extended beyond ~1-2 hr, fat again becomes the dominant fuel. In addition, fat oxidation contributes energy during recovery from exercise. The purpose of this Sports Science Exchange article is to briefly review the latest information on the regulation of fat use during exercise. This topic has been examined in more detail by several authors in the past (Glatz et al., 2010; Kiens, 2006; Sahlin, 2009; Spriet, 2012, 2014). A second Sports Science Exchange article on fat metabolism examines nutritional and training strategies that can affect fat oxidation rates (Randell & Spriet, 2020).
FAT UTILIZATION DURING EXERCISE
During exercise that is aerobic in nature (requiring less energy than 100% VO2max), the oxidation of CHO and fat provides the energy for contracting skeletal muscles. Other potential fuels like amino acids can also contribute energy but this contribution is usually small in well fed subjects. The major substrates in muscle (endogenous) for aerobic energy production are glycogen and intramuscular triglyceride (IMTG) and from outside the cell (exogenous) are blood glucose (derived from liver glycogenolysis and gluconeogenesis, and from the gut when CHO is ingested) and free fatty acids (FFA) derived from adipose tissue triglyceride (TG) stores. The reliance on these four sources of fuel was measured in well-trained young male cyclists at varying exercise intensities (Figure 1) using indirect calorimetry, stable isotope techniques and skeletal muscle biopsies (Romijn et al., 1993). Measurements were made during the final 30 min of a 2 h cycle at 25 and 65% VO2max and the final 10 min of a 30 min cycle at 85% VO2max. A second study with very similar results was published by van Loon et al. in 2001.
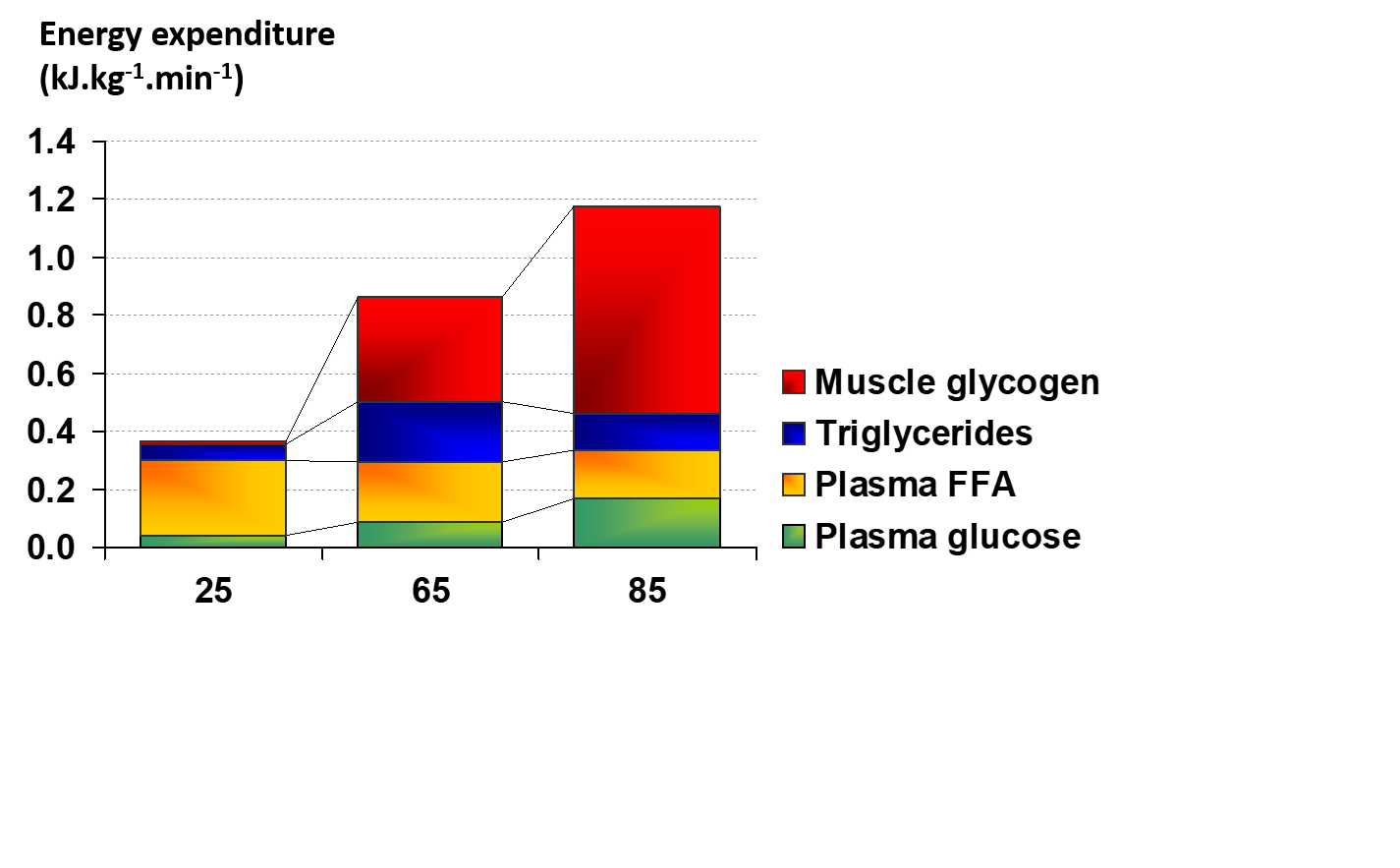 These data provided several important insights regarding fuel utilization with increasing exercise intensity. At 25% VO2max, glucose and FFA were transported into muscle at a rate that provided the required fuel, with FFA the dominant fuel at this low intensity (Figure 1). When the exercise intensity was increased to 65% VO2max (moderate intensity), the contribution from exogenous FFA was maintained, the exogenous glucose contribution increased, and significant amounts of muscle glycogen and IMTG were also used (Romijn et al., 1993). The contribution of fat reached its max at this moderate power output and the total contribution from fat and CHO was about 50/50 (Figure 1). Additional research has demonstrated that an exercise intensity of ~60-65% elicits maximal fat oxidation rates (Achten et al., 2002; Randell et al., 2017). When moving to 85% VO2max (high intensity), the contribution from FFA and IMTG decreased, reliance on blood-borne glucose increased, and the use of muscle glycogen became the dominate provider of fuel. To summarize, CHO oxidation, mainly from muscle glycogen, dominated at the higher exercise intensities and fat oxidation was more important at low and moderate intensities.
These data provided several important insights regarding fuel utilization with increasing exercise intensity. At 25% VO2max, glucose and FFA were transported into muscle at a rate that provided the required fuel, with FFA the dominant fuel at this low intensity (Figure 1). When the exercise intensity was increased to 65% VO2max (moderate intensity), the contribution from exogenous FFA was maintained, the exogenous glucose contribution increased, and significant amounts of muscle glycogen and IMTG were also used (Romijn et al., 1993). The contribution of fat reached its max at this moderate power output and the total contribution from fat and CHO was about 50/50 (Figure 1). Additional research has demonstrated that an exercise intensity of ~60-65% elicits maximal fat oxidation rates (Achten et al., 2002; Randell et al., 2017). When moving to 85% VO2max (high intensity), the contribution from FFA and IMTG decreased, reliance on blood-borne glucose increased, and the use of muscle glycogen became the dominate provider of fuel. To summarize, CHO oxidation, mainly from muscle glycogen, dominated at the higher exercise intensities and fat oxidation was more important at low and moderate intensities.
In a similar experiment with young well-trained women (Romijn et al., 2000), fuel utilization findings at 25, 65 and 85% VO2max were essentially identical to those of the men (Romijn et al., 1993). In less well-trained or untrained subjects, the reliance on CHO is higher at moderate and higher power outputs, and exercise at power outputs of ~60% VO2max and above cannot be sustained for as long as trained subjects, even though the absolute power outputs are much lower (Coggan et al., 1995a, b; Howlett et al., 1998).
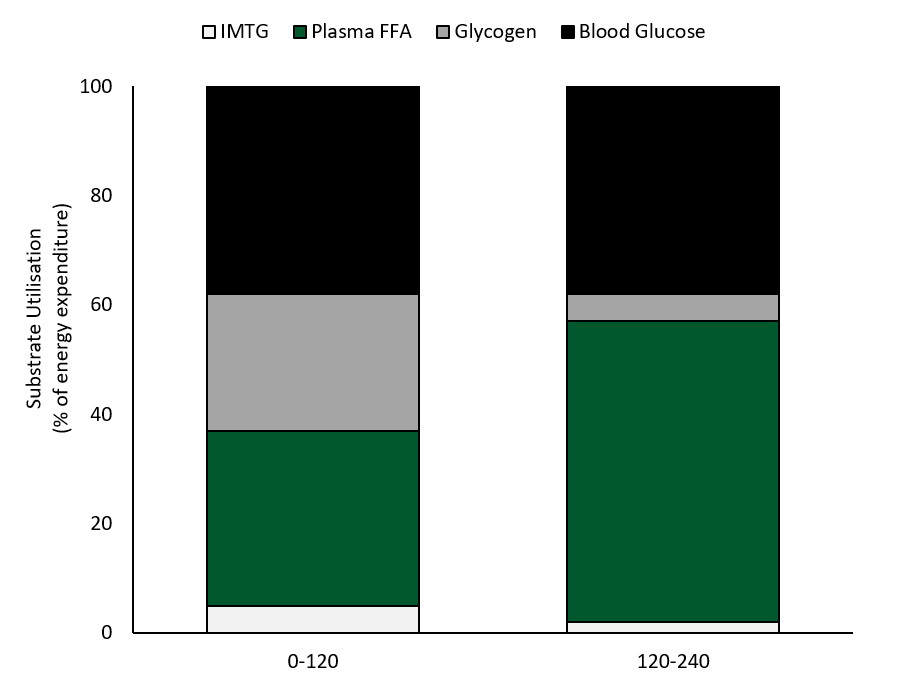
Watt et al. (2002)had well-trained cyclists ride for 4 h at 55% VO2max and examined fuel use with indirect calorimetry and measurements of skeletal muscle IMTG and glycogen use from biopsy samples (Figure 2). CHO oxidation dominated in the first 2 h accounting for ~63% of the energy provision, with glycogen providing 45% and blood glucose 55% of the CHO used. Fat accounted for 37% of the fuel use in the first 2 h, with plasma FFA oxidation providing 90% and IMTG use only 10% (Figure 2). Plasma glucose provided slightly more of the blood-borne energy than plasma FFA in the initial 2 h of exercise. However, fat oxidation increased over time and became the dominant fuel at ~2 h as CHO oxidation steadily decreased. In hours 2-4, fat oxidation accounted for 58% of the fuel with virtually all the substrate provided by plasma FFA. There was a steady increase in plasma [FFA] from ~0.2 mmol/L at rest to ~0.9 at 2 h and 1.66 + 0.32 mmol/L at 4 h (Watt et al., 2002). While CHO oxidation decreased in the final 2 h, it supplied 42% of the energy and most of the fuel (85%) was derived from plasma glucose uptake. Blood glucose was reasonably well maintained over the 4 h of exercise period and was 4.9 + 0.3 mmol/L at rest, 5.1+ 0.3 mmol/L at 1 h, and decreased to 4.0 + 0.3 mmol/L at 4 h. Blood lactate levels were very low throughout the entire cycle, but plasma glycerol increased steadily from 119 + 18 umol/L at rest to 701 + 58 umol/L at 4 h (from adipose and IMTG breakdown), providing the liver with substrate for gluconeogenesis.
In the final 2 h of exercise at ~57% VO2max blood-borne fuel accounted for 92% of the oxidative energy with FFA contributing 60% and blood glucose 40%. These data clearly demonstrated the shift away from intramuscular fuel use in the first half of prolonged exercise and the increased reliance on blood borne FFA and glucose in the latter half of the 4 h ride, with plasma FFA becoming the dominant fuel.
 REGULATION OF FAT METABOLISM DURING EXERCISE
REGULATION OF FAT METABOLISM DURING EXERCISE
Skeletal muscle has well-developed metabolic pathways to deal with the sudden and continued demand for energy or adenosine triphosphate (ATP) during exercise. Much of the resynthesis of ATP from the by-products of ATP degradation, adenosine diphosphate (ADP) and inorganic phosphate (Pi), occurs through oxidative (“aerobic”) phosphorylation in the electron transport chains (ETC) of the mitochondria. The ETC requires reducing equivalents from the metabolism of CHO and fat, ADP and Pi, and oxygen to regenerate ATP (Figure 3).
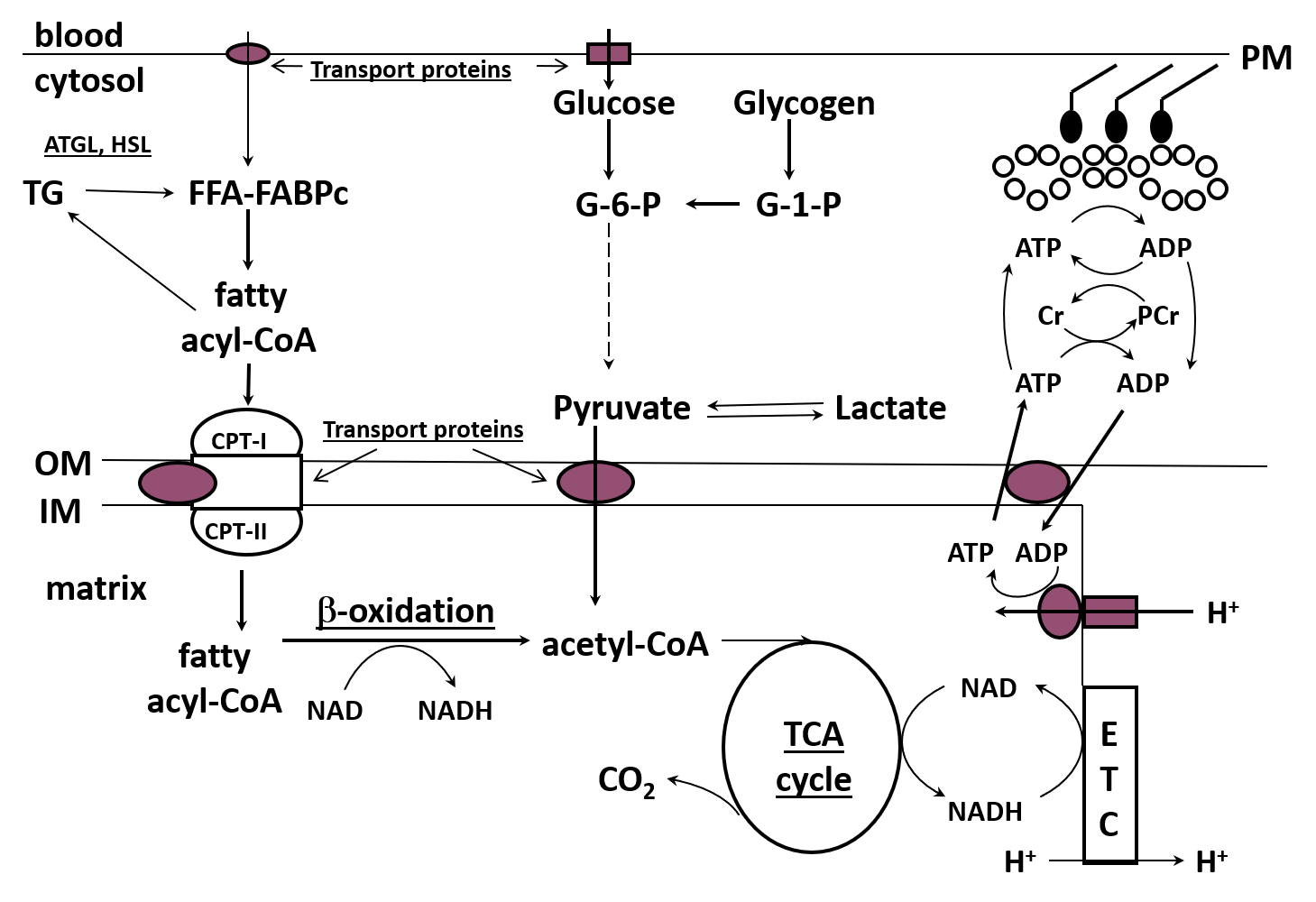 Research in the past 15-20 years has demonstrated that the regulation of fat metabolism is complex and involves many sites of control, including the transport of fat into the muscle cell, the binding to a protein chaperone and movement of fat in the cytoplasm, the regulation of IMTG synthesis and breakdown in the cytoplasm, and the transport of fat into the mitochondria (Figure 3). The fat then enters the beta-oxidation pathway which produces reducing equivalents (NADH, FADH2) and acetyl-CoA which are used in the ETC to generate energy and the tricarboxylic acid (TCA) cycle to produce more reducing equivalents, respectively. The beta-oxidation pathway does not appear to be externally regulated and may simply be activated by substrate delivery, but there is additional regulation which occurs at three sites in the TCA cycle (Kiens, 2006; Sahlin, 2009; Spriet, 2012, 2014). It is generally accepted that, in the presence of ample reducing equivalents and oxygen, the increase in ADP at the onset of exercise increases the ETC flux to resynthesize ATP (Holloway, 2017; Sahlin, 2009).
Research in the past 15-20 years has demonstrated that the regulation of fat metabolism is complex and involves many sites of control, including the transport of fat into the muscle cell, the binding to a protein chaperone and movement of fat in the cytoplasm, the regulation of IMTG synthesis and breakdown in the cytoplasm, and the transport of fat into the mitochondria (Figure 3). The fat then enters the beta-oxidation pathway which produces reducing equivalents (NADH, FADH2) and acetyl-CoA which are used in the ETC to generate energy and the tricarboxylic acid (TCA) cycle to produce more reducing equivalents, respectively. The beta-oxidation pathway does not appear to be externally regulated and may simply be activated by substrate delivery, but there is additional regulation which occurs at three sites in the TCA cycle (Kiens, 2006; Sahlin, 2009; Spriet, 2012, 2014). It is generally accepted that, in the presence of ample reducing equivalents and oxygen, the increase in ADP at the onset of exercise increases the ETC flux to resynthesize ATP (Holloway, 2017; Sahlin, 2009).
The discovery of proteins that assist in transporting fat across the plasma and mitochondrial membranes, the ability of these proteins to translocate to the membranes during exercise, and the new roles of adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) in regulating skeletal muscle lipolysis are examples of recent discoveries in the regulation of fat metabolism.
Fat Transport Across Membranes
It is now clear that the majority of FFA that moves into muscle cells during exercise is not due to simple diffusion, but is facilitated across the muscle membrane and t-tubules via protein-mediated transport systems (Bonen et al., 2000; Holloway et al., 2006; Stefanyk et al., 2012). This followed earlier work demonstrating that FFA movement across the muscle membrane was a saturable process involving proteins (Turcotte et al., 1991, 2000). These transport proteins include the plasma membrane fatty acid binding protein (FABPpm), fatty acid translocase (FAT/CD36) and fatty acid transport (FATP) proteins. Once in the cytoplasm, FFA are bound to a protein chaperone (cytoplasmic fatty acid transport protein) to be transported for storage as IMTG or delivery to the mitochondria for oxidation (Figure 3).
The FFA (from outside the cell and/or released from IMTG) must be transported across the mitochondrial membranes with the help of the carnitine palmitoyl transferase I (CPT I) system and fat transport proteins (mainly FAT/CD36) (Holloway et al., 2006; Smith et al., 2012b). During acute exercise, fat transport proteins are also moved to the muscle membrane (mainly FABPpm) and mitochondrial membranes (mainly FAT/CD36) to help bring fat into the cell and into the mitochondria but this occurs over a slower time course (~15-30 min) than the movement (s to min) of the glucose transporter (GLUT4) (Holloway et al. 2006; Bradley et al., 2012). It has been proposed that FAT/CD36 is located on the outer mitochondrial membrane and in some unexplained manner, facilitates the delivery of long chain fatty acids to the acyl-CoA synthase enzyme to be activated so they can interact with the CPT complex and move into the mitochondria (Smith et al., 2012a, b).
In human skeletal muscle, exercise training increased FABPpm content on the muscle membranes and increased the FAT/CD36 content on the mitochondrial membranes to a greater extent than the increase in mitochondrial volume (Bradley et al., 2012; Talanian et al., 2010). These adaptations are consistent with the increased capacity to oxidize fat following exercise training (Holloway & Spriet, 2009; Holloszy & Coyle, 1984; Perry et al., 2008).
Intramuscular Triglyceride Breakdown
The breakdown of IMTG in muscle can also provide FFAs for oxidation during low and moderate intensity exercise and sprint and resistance exercise (Romijn et al., 1993; Shepherd et al., 2013, 2014; Stellingwerff et al., 2007). Exercise training also increases the IMTG content and the reliance on IMTG during exercise (Goodpaster et al., 2001; Shepherd et al., 2013, 2014). The key enzymes involved in regulating lipolysis in skeletal muscle are ATGL, HSL and monoglyceride lipase (MGL) that sequentially remove a fatty acid (FA) from the IMTG stored in lipid droplets (Figure 3). ATGL and HSL are highly regulated, while MGL is not (Alsted et al., 2009; Prats et al., 2006). Other factors also play a role in IMTG breakdown, including lipid droplet size, droplet localization and the fact that lipid droplets are surrounded by a protein (perilipins) coating (Jevons et al., 2020; MacPherson & Peters 2015; Prats et al., 2006).
Mitochondrial Fat Metabolism
Once inside the mitochondria, FAs enter the beta oxidation pathway with the formation of acetyl-CoA and reducing equivalents (NADH, FADH2). To date, there has been no concrete evidence that metabolic regulation occurs in the beta-oxidation pathway – it simply responds to the provision of substrate (Sahlin, 2009). The reducing equivalents generated in the beta-oxidation pathway enter the ETC and together with other substrates, oxygen and free ADP and Pi, result in the generation of ATP. The acetyl-CoA produced in the beta-oxidation pathway enters the TCA cycle and this pathway specializes in producing more reducing equivalents and accepts acetyl-CoA from both CHO and fat (and to a minor extent other fuels). During aerobic exercise, the isocitrate and alpha-ketoglutarate dehydrogenase enzymes and a third enzyme, citrate synthase, are activated increasing flux through the TCA cycle (Sahlin, 2009). The combination of reducing equivalents produced in the beta-oxidation and TCA pathways and the long chain nature of FAs like palmitate and oleate results in a large amount of ATP production in the ETC (Figure 3).
Overall Regulation of Fat Use as a Fuel
The increase in fat use during exercise is regulated by many of the same signals as CHO metabolism. The increase in Ca2+ at the onset of exercise activates the key enzymes and processes involved in regulating IMTG degradation (as does epinephrine), the movement and docking of fat transport proteins at the muscle membrane and the TCA cycle enzymes in the mitochondria (Hargreaves & Spriet, 2017; Sahlin, 2009). Local factors related to the energy status of the cell including increases in free ADP and adenosine monophosphate (AMP), and activation of AMP kinase (AMPK) also contribute to the regulation at several sites (Fentz et al., 2015; Jain et al., 2009, 2015), while factors including malonyl-CoA, pH, and carnitine are involved in the regulation of fat transport across the mitochondrial membranes (Petrick & Holloway, 2019; Smith et al., 2012a).
Exercise Training
Given that the beta oxidation and TCA pathways exist in the mitochondria, the mitochondrial volume of the muscle cell determines the overall capacity to oxidize fat during exercise. The classic work by Holloszy and colleagues demonstrated that aerobic and high intensity intermittent exercise training increased mitochondrial volume in rat and human skeletal muscle (Holloszy, 1967; Holloszy et al., 1970; Mole et al., 1971). While adequate supplies of oxygen, ADP, Pi and reducing equivalents from fat are required for mitochondrial ATP production, the mitochondrial volume or machinery available for generating it is also important. Therefore, a greater mitochondrial volume following training provides the means for a greater capacity to produce NADH from fat, and hence more ATP in the ETC (Holloway & Spriet, 2009; Perry et al., 2008). Training also results in the classic finding that the reliance on fat as a fuel increases at a given absolute submaximal power output following training (Holloszy & Coyle, 1984; Perry et al., 2008). Other important consequences of exercise training include an improved ability to deliver and take up FFA into the muscle (Turcotte et al., 1992), increased storage of IMTG (Goodpaster et al., 2001), upregulation of ATGL activity, and increases in the fat transport protein content at the muscle and mitochondrial membranes (Bradley et al., 2012; Talanian et al., 2010).
Decreased Reliance on Fat at Higher Exercise Intensities
During intense endurance events athletes are often competing at ~85-95% VO2max and fuel use shifts away from fat and to CHO (Figure 1). From a performance point of view this fuel shift makes sense as the energy yield from CHO oxidation is ~7% more efficient than from fat. Research has now identified several sites where fat metabolism is down-regulated at high aerobic exercise intensities, including decreased FFA release from adipose tissue, less transport to the muscles and therefore less FFA transport into muscle, decreased activation of HSL and possibly ATGL and less IMTG breakdown, inhibition of CPT I activity due to small decreases in muscle pH, decreased CPT I sensitivity to carnitine, and possibly low levels of cytoplasmic carnitine, reducing mitochondrial membrane transport (Petrick & Holloway 2019; Smith et al., 2012a; Spriet, 2014).
FAT METABOLISM DURING RECOVERY FROM EXERCISE
Relatively little research attention has been directed to the importance of fat as a fuel in skeletal muscle during rested recovery from prolonged exercise or during the rest or low power output periods between bouts of high intensity exercise. After prolonged exercise, the respiratory exchange ratio (RER) may not accurately predict fat use in skeletal muscle as the metabolic rate of the muscle is lower and does not dominate the gas exchange data as in exercise. In addition, other metabolic processes involving the use of carbon dioxide may affect the measured RER. During intermittent exercise, where the rest periods are often short, the attainment of a steady state to measure gas exchange and the use of RER to predict fat use are also difficult.
Despite these issues, studies have indicated that fat oxidation is elevated after exercise when compared to a resting control situation (Henderson et al., 2007; Malatesta et al., 2009). To circumvent the RER issues during recovery Henderson et al. (2007) measured whole body FA oxidation with 13C-palmitate for 3 h following exercise for 90 min at 45% VO2max, and following exercise for 60 min at 65% VO2max and a time-matched sedentary control in moderately active men and women. Plasma FA oxidation was elevated above rest for the entire 3 h recovery period in both sexes. There was no difference between the two exercise bouts, but total FA oxidation was elevated more in men than women. Several other studies have reported elevated fat oxidation in trained men and women for several hours post glycogen-depleting exercise, based on RER measures and despite consuming food rich in CHO following exercise. In most studies, IMTG stores did not decrease and often increased during 18-30 h recovery periods (Decombaz et al., 2001; Kimber et al., 2003; Larson-Meyer et al., 2002), although one study reported a net decrease in IMTG following 18 h of recovery in well-trained males (Kiens & Richter, 1998). These data suggest that plasma FFA and possibly very low-density lipoproteins are likely to be the important fat fuels for aerobic energy in the immediate recovery from exercise.
Maletesta et al. (2009) examined lipid oxidation during 3 h of recovery from high intensity submaximal intermittent exercise (1 min at 80% VO2max with 1 min of active recovery at 40% VO2max), 60 min of exercise at 45% VO2max, and a time-matched resting control trial in active young men. The increase in total substrate oxidation and fat oxidation following the two iso-energetic exercise trials was the same in the recovery period and higher than the control trial. This occurred despite less fat oxidation during the intermittent exercise trial compared to the constant power output trial, suggesting that the total energy expenditure dictated the fat oxidation rate during the 3 h recovery period. Clearly more research examining fuel utilization during recovery from exercise is warranted and improved methods to estimate fat and CHO use in the short rest or lower power output periods between high intensity exercise bouts are needed.
CONCLUSIONS
Fat is an important fuel for low and moderate exercise intensity, especially if the exercise is prolonged. The regulation of fat metabolism in skeletal muscle during exercise is complex and involves many sites of control. The activation of fat oxidation at the onset of exercise is slower than CHO and is designed for long-term low to moderate intensity exercise, as the capacity is much larger than CHO. Several locations of down regulation of fat metabolism have been identified to explain the decrease in fat oxidation during high intensity aerobic exercise. More research is needed to determine the importance of fat as a fuel during the recovery from a single bout of exercise and the rest and lower power outputs that occur between bouts of high intensity exercise common to stop-and-go sports.
Rebecca Randell is employed by the Gatorade Sports Science Institute, a division of PepsiCo, Inc. The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Achten, J., M. Gleeson, and A.E. Jeukendrup (2002). Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 34: 92-97.
Alsted, T.J., L. Nybo, M. Schweiger, C. Fledelius, P. Jacobsen, R. Zimmermann, R. Zechner, and B. Kiens (2009). Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. 296:E445-453.
Bonen, A., J.J. Luiken, Y. Arumugam, J.F. Glatz, and N.N. Tandon (2000). Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J. Biol. Chem. 275:14501-14508.
Bradley, N.S., L.A. Snook, S.S. Jain, G.J.F. Heigenhauser, A. Bonen, and L. . Spriet (2012). Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am. J. Physiol. 302:E183-189.
Coggan, A.R., C.A. Raguso, B.D. Williams, L.S. Sidossis, and A. Gastaldelli (1995a). Glucose kinetics during high-intensity exercise in endurance-trained and untrained humans. J. Appl. Physiol. 78:1203-1207.
Coggan, A.R., S.C. Swanson, L.A. Mendenhall, D.L. Habash, and C.L. Kien (1995b). Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am. J. Physiol. 268:375-383.
Decombaz, J., B. Schmitt, M. Ith, B. Decarli, P. Diem, R. Kreis, H. Hoppeler, and C. Boesch (2001). Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am. J. Physiol. 281:R760-R769.
Fentz. J., R. Kjobsted, J.B. Birk, J. Jeppesen, K. Thorsen, P. Schjerling, B. Kiens, N. Jessen, B. Viollet, and J.F. Wojtaszewski (2015). AMPKα is critical for enhancing skeletal muscle fatty acid utilization during in vivo exercise in mice. FASEB J. 29:1725-1738.
Goodpaster, B.H., J. He, S. Watkins, and D.E. Kelley (2001). Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86:755–5761.
Glatz, J.F., J.J. Luiken, and A. Bonen (2010). membrane fatty acid transporters as regulators of lipid metabolism; Implications for metabolic disease. Physiol. Rev. 90:367-417.
Hargreaves, M., and L.L. Spriet (2017). Exercise metabolism: Fuels for the fire. In: The Biology of Exercise. Zierath, J.R., M.J. Joyner, and J.A. Hawley (eds). Cold Spring Harbor Laboratory Press. Cold Spring Harbor, USA. pp. 57-72.
Henderson, G.C., J.A. Fattor, M.A. Horning, N. Faghihnia, M.L. Johnson, T.L. Mau, M. Luke-Zeitoun, and G.A. Brooks (2007). Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol. 584:963-981.
Holloway, G.P. (2017). Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med. 47:S13-S21.
Holloway, G.P., and L.L. Spriet (2009). Skeletal muscle metabolic adaptations to training. In: The IOC Textbook of Science in Sport. R.J. Maughan (Ed): O. Wiley-Blackwell, UK. pp. 70-83.
Holloway, G.P., V. Bezaire, G.J.F. Heigenhauser, N.N. Tandon, J.F. Glatz, J.J. Luiken, A. Bonen, and L. L. Spriet (2006). Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol 571: 201-210.
Holloszy, J.O. (1967). Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 242:2278–2282.
Holloszy, J.O., and E.F. Coyle (1984). Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. 56:831–838.
Holloszy, J.O., L.B. Oscai, I.J. Don, and P.A. Mole (1970) Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem. Biophys. Res. Commun. 40:1368–1373.
Howlett, R.A., M.L. Parolin, D.J. Dyck, E. Hultman, N.L. Jones, G.J.F. Heigenhauser, and L.L. Spriet (1998). Regulation of skeletal muscle glycogen phosphorylase and pyruvate dehydrogenase at varying power outputs. Am. J. Physiol. 275:R418-R425.
Jain, S.S., A. Chabowski, L.A. Snook, R.W. Schwenk, J.F. Glatz, J.J. Luiken, and A. Bonen (2009). Additive effects of insulin and muscle contraction on fatty acid transport and fatty acid transporters, FAT/CD36, FABPpm, FATP1, 4 and 6. FEBS Lett. 583:2294-2300.
Jain, S.S., J.J. Luiken, L.A. Snook, X.X. Han, G.P. Holloway, J.F. Glatz, and A. Bonen (2015). Fatty acid transport and transporters in muscle are critically regulated by Akt2. FEBS Lett. 589:2769-2775.
Jevons, E.F.P., K.D. Gejl, J.A. Strauss, N. Ortenblad, and S.O. Shepherd (2020). Skeletal muscle lipid droplets are resynthesized before being coated with perilipin proteins following prolonged exercise in elite male triathletes. Am. J. Physiol. 318:E357-E370.
Kiens, B. (2006). Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86:205-243.
Kiens, B., and E.A. Richter (1998). Utilization of skeletal muscle triacylglcerol during postexercise recovery in humans. Am. J. Physiol. 275:E332-E337.
Kimber, N.E., G.J.F. Heigenhauser, L.L. Spriet, and D.J. Dyck (2003). Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J. Physiol. 548:919-927.
Larson-Meyer, D.E., B.R. Newcomer, and G.R. Hunter (2002). Influence of endurance running and revovery diet on intramyocellular lipid content in women: a 1H NMR study. Am. J. Physiol. 282:E95-E106.
MacPherson, R.E., and S.J. Peters (2015). Piecing together the puzzle of perilipin proteins and skeletal muscle lipolysis. Appl. Physiol. Nutr. Metab. 40:641-651.
Malatesta, D., C. Werlen, S. Bulfaro, X. Cheneviere, and F. Borrani (2009). Effect of high-intensity interval exercise on lipid oxidation during postexercise recovery. Med. Sci. Sports Exerc. 41:364-374.
Mole, P.A., L.B. Oscai, and J.O. Holloszy (1971). Adaptation of muscle to exercise. Increase in levels of palmityl CoA synthetase, carnitine palmityltransferase, and palmityl CoA
dehydrogenase, and in the capacity to oxidize fatty acids. J. Clin. Invest. 50:2323–2330.
Perry, C.G., G.J.F. Heigenhauser, A. Bonen, and L.L. Spriet (2008). High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl. Physiol. Nutr. Metab. 33:1112-1123.
Petrick, H.L., and G.P. Holloway (2019). High intensity exercise inhibits carnitine palmitoyltransferase-I sensitivity to l-carnitine. Biochem. J. 476:547-558.
Prats, C., M. Donsmark, K. Qvortrup, C. Londos, C. Sztalryd, C. Holm, H. Galbo, and T. Ploug (2006). Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J. Lipid Res. 47:2392-2399.
Randell, R.K., and L.L. Spriet (2020). Factors affecting fat oxidation rates in athletes. Sports Science Exchange #206 .
Randell, R.K., I. Rollo, T.J. Roberts, K J. Dalrymple, A.E. Jeukendrup, and J.M. Carter (2017). Maximal fat oxidation rates in an athletic population. Med. Sci. Sports Exerc. 49:133-140.
Romijn, J.A., E.F. Coyle, L.S. Sidossis, A. Gastaldelli, J.F. Horowitz, E. Endert, and R.R. Wolfe (1993). Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265:E380-391.
Romijn, J.A., E.F. Coyle, L.S. Sidossis, J. Rosenblatt, and R.R. Wolfe (2000). Substrate metabolism during different exercise intensities in endurance-trained women. J. Appl. Physiol. 88:1707-1714.
Sahlin, K. (2009). Control of lipid oxidation at the mitochondrial level. Appl. Physiol. Nutr. Metab. 34:382-388.
Shepherd, S.O., M. Cocks, K.D. Tipton, A.M. Ranasinghe, T.A. Barker, J.G. Burniston, A.J.M. Wagenmakers, and C.S. Shaw (2013). Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J. Physiol. 591:657-675.
Shepherd, S.O., M. Cocks, K.D. Tipton, O.C. Witard, A.M. Ranasinghe, T.A. Barker, A.J.M. Wagenmakers, and C.S. Shaw (2014). Resistance training increases skeletal muscle oxidative capacity and net intramuscular triglyceride breakdown in type I and II fibers of sedentary males. Exp. Physiol. 99:894-908.
Smith, B.K., C.G. Perry, T.R. Koves, D.C. Wright, J.C. Smith, P.D. Neufer, D.M. Muoio, and G.P. Holloway (2012a). Identification of a novel malonyl-CoA IC50 for CPT-1: Implications for predicting in vivo fatty acid oxidation rates. Biochem. J. 448:13–20.
Smith, B.K., A. Bonen and G.P. Holloway (2012b). A dual mechanism of action for skeletal muscle FAT/CD36 during exercise. Exerc. Sport Sci. Rev. 40:211-217.
Spriet, L.L. (2012). The metabolic systems: Lipid metabolism. In: Farrell, P.A., M.J. Joyner, and V.J. Caiozzo, (eds). Advanced Exercise Physiology, 2nd Ed. W. Lippincott, Wilkins. Philadelphia, PA, USA. pp. 392-407.
Spriet, L.L. (2014). New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 44:S87-S96.
Stefanyk, L.E., A. Bonen, and D.J. Dyck (2012). Insulin and contraction-induced movement of fatty acid transport proteins to skeletal muscle transverse tubules is distinctly different
than to the sarcolemma. Metabolism 61:1518–1522.
Stellingwerff, T., H. Boon, R.A. Jonkers, J.M. Senden, L.L. Spriet, R. Koopman, and L.J. van Loon (2007). Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am. J. Physiol. 292:E1715-1723.
Talanian, J.L., G.P. Holloway, L.A. Snook, G.J.F. Heigenhauser, A. Bonen, and L.L. Spriet (2010). Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am. J. Physiol. 299:E180-188.
Turcotte, L., B. Kiens, and E.A. Richter (1991). Saturation kinetics of palmitate uptake in perfused skeletal muscle. FEBS Lett. 279:327–329.
Turcotte, L.P., E.A. Richter, and B. Kiens (1992). Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am. J. Physiol. 262:E791–E799.
Turcotte, L.P., J.R. Swenberger, M.Z. Tucker, A.J. Yee, G. Trump, J.J. Luiken, and A. Bonen
(2000). Muscle palmitate uptake and binding are saturable and inhibited by antibodies to FABP(PM). Mol. Cell. Biochem. 210:53–63.
van Loon, L.J., P.L. Greenhaff, D. Constantin-Teodosiu, W.H. Saris and A.J. Wagenmakers (2001). The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 536: 295-304.
Watt, M.J., G.J.F. Heigenhauser, D.J. Dyck, and L.L. Spriet (2002). Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 541:969-978.
Figure Legends
Figure 1. Fuel use during exercise at 25, 65, and 85 VO2 max (reproduced from Romijn et al., 1993).
Figure 2. Fuel use during 4 h of exercise at ~57% VO2 max (reproduced from Watt et al., 2002). FFA, free fatty acids; IMTG, intramuscular triglyceride.
Figure 3. Schematic of fat metabolism in skeletal muscle. FFA, free fatty acids; ALB, albumin; BF, bllod flow; ATGL, adipose triglyceride lipase; HSL, hormone sensitive lipase; FABPc, cytoplasmic fatty acid binding protein; CPT 1 and II, carnitine palmitoyl tranferase iI and II; OM, outer membrane; IM, inner membrane; TCA, tricarbolylic acid cycle; ETC, electron transport chain; G-6-P, glucose 6-phophate; G-1-P, glucose 1-phosphate; Cr, creatine; PCr, phosphocreatine. Underlines denote sites of fat regulation.








