Fish Oil for Healthy Aging - Potential Applications for Master Athletes

KEY POINTS
- After approximately 50 years of age, people begin to lose skeletal muscle mass and strength, a process mitigated by exercise training. Older adults who regularly engage in exercise training are referred to as master athletes and often display phenotypic characteristics similar to their younger, untrained counterparts.
- A key aspect supporting the adaptive response of skeletal muscle to exercise training is adequate nutrition, with the majority of research in the sport and exercise science domains focusing on the role of protein and carbohydrate ingestion. However, recent research in the clinical setting in older adults indicates a potential ergogenic effect of long chain n-3 polyunsaturated fatty acids (LC n-3 PUFAs).
- In untrained older adults, LC n-3 PUFA intake (~3–5 g/d) enhances rates of muscle protein synthesis with amino acid and insulin infusion, promotes gains in muscle size, and potentiates strength gains with resistance exercise training. However, not all studies have shown a positive effect, and it is therefore unclear if LC n-3 PUFA ingestion is ergogenic for master athletes.
- There is no evidence that LC n-3 PUFA intake is ergogenic for endurance exercise performance or recovery in humans of any age or training status.
- Current population intake recommendations are 250–500 mg eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) per day. There is emerging evidence that EPA+DHA intakes above the recommended amount improves LC n-3 PUFA status and may confer health benefits in older adults; however, further research is required.
- Oily fish is an excellent source of LC n-3 PUFAs and supplementation is a viable option for individuals who are unable or do not wish to consume oily fish.
INTRODUCTION
After approximately 50 years of age, people begin to lose skeletal muscle mass and strength that can lead to a disease known as sarcopenia. Sarcopenia results in reduced capacity to produce force and is a primary risk factor for the development of numerous negative health outcomes (Janssen, 2006). It is thought that engaging in high levels of physical activity and/or exercise training can mitigate some of the negative consequences of sarcopenia (McKendry et al., 2020). In this regard, there is evidence that older adults who regularly engage in exercise training— or master athletes— display similar skeletal muscle biochemical and performance characteristics compared with younger healthy persons (McKendry et al., 2018). These findings not only highlight the importance of exercise training to promote athletic performance in master athletes but also to support healthy aging in the general population.
A key factor supporting both exercise performance and recovery from exercise is appropriate nutritional intake. For instance, carbohydrate ingestion is required to provide the fuel necessary to support energy needs during exercise and restore levels of muscle glycogen during the recovery period (Burke et al., 2011). Protein intake, particularly high-quality protein rich in amino acids, is essential for the remodelling, repair, and growth of damaged skeletal muscle tissue (McGlory et al., 2018). However, the role of fatty acids in supporting the adaptive response to exercise training has received comparably less attention. Traditionally associated with improved cardiovascular health, there is now emerging evidence from the clinical setting that ingestion of long chain n-3 polyunsaturated fatty acids (LC n-3 PUFAs) potentiates skeletal muscle anabolism in older adults (Smith et al., 2015). These findings are complemented by reports in younger persons indicating a potential role for LC n-3 PUFAs in mitigating indices of muscle soreness and inflammation in response to damaging exercise (Anthony et al., 2021). In this Sports Science Exchange (SSE) article, we will build on previous SSE articles focused primarily on younger persons (Rockwell & Ritz 2021; Witard & Davis 2021) to explore the potential for LC n-3 PUFAs to enhance exercise performance and recovery in master athletes. We will also provide recommendations for practical application.
WHAT ARE LC n-3 PUFAs?
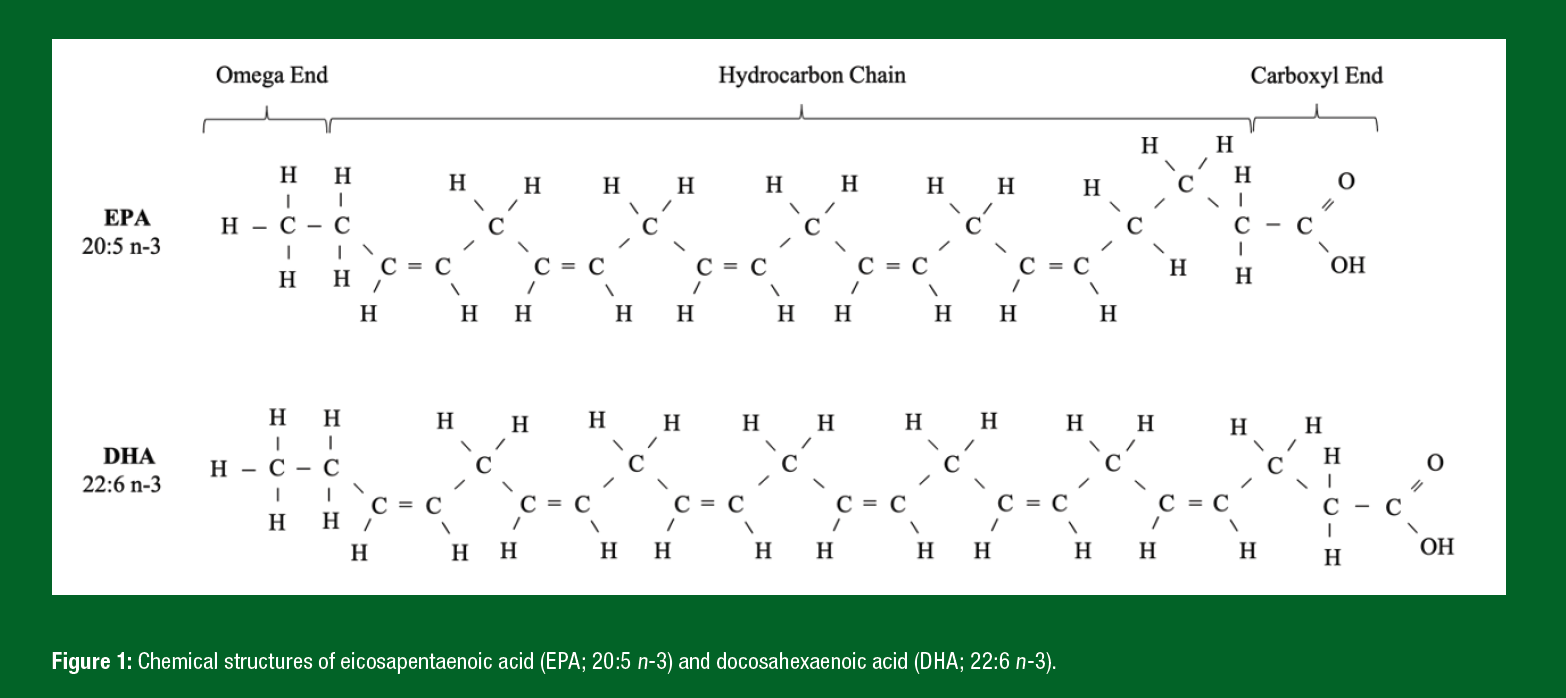
LC n-3 PUFAs are a class of biologically active fatty acids, sometimes referred to as omega-3 fatty acids, consisting of a hydrocarbon chain with a carboxylic acid group at one end and a methyl (omega) group at the other. The n-3 nomenclature refers to the position of the first double carbon bond away from the methyl end. The most well-studied LC n-3 PUFAs are eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3) (Figure 1). Population intake recommendations for LC n-3 PUFA (250–500 mg EPA+DHA/d) can be achieved with the consumption of 1 to 2 portions (100–140 g/portion) of oily fish per week (EFSA, 2005; The Scientific Advisory Committee on Nutrition, 2004; US Department of Health and Human Services and US Department of Agriculture, 2015). These fatty acids play numerous roles in cellular function, such as serving as the substrate for the production of anti-inflammatory resolving mediators as well as suppressing the expression of pro-inflammatory molecules. LC n-3 PUFAs are also key components of phospholipids that make up our membranes (McGlory et al., 2019). Given the important role of LC n-3 PUFAs in cellular health and function, their impact on skeletal muscle in response to exercise training has become a topic of increased scientific research.
ARE LC n-3 PUFAs ERGOGENIC FOR MASTER ATHLETES?
Skeletal Muscle Anabolism
Initial evidence that LC n-3 PUFAs altered skeletal muscle anabolism emanated from work in steers. The authors demonstrated that in response to an LC n-3 PUFA-rich fish oil infusion, there was an increase in the EPA and DHA composition of skeletal muscle phospholipid membranes and a doubling of whole-body amino acid disposal, indicative of protein accretion (Gingras et al., 2007). Later, Smith et al. (2011) reported potentiated rates of muscle protein synthesis (MPS) in response to a hyperaminoacidemic-hyperinsulinemic clamp following 8 weeks of 1.66 g/d EPA and 1.50 g/d DHA supplementation in older women and men. The finding of potentiated MPS rates in response to amino acid and insulin provision following LC n-3 PUFA intake is especially intriguing given that, despite high training volumes, master athletes still exhibit age-related declines in MPS rates similar to that of their untrained counterparts (McKendry et al., 2019). Further studies have also reported improvements in muscle size and strength with 6 months of 1.66 g/d EPA and 1.50 g/d DHA supplementation in older persons (Smith et al., 2015) as well as improved muscle strength with LC n-3 PUFA intake in response to 18 weeks of resistance exercise training in older women (Da Boit et al., 2017) (see Witard & Davis 2021 for further discussion).
While there is growing evidence that LC n-3 PUFA intake may exert a positive impact towards skeletal muscle, not all studies have shown such an effect. Indeed, a recent study by Murphy et al. (2021) observed no beneficial effects of 6 months of supplementation with 1.6 g/d EPA + 2.3 g DHA/d, provided as part of a mixed macronutrient drink containing leucine-enriched protein, on appendicular lean mass, strength, or physical performance in older adults who had low muscle mass at baseline. Furthermore, a large clinical trial in over 2,000 older adults reported no impact of 3 years of supplementation with 0.3 g EPA/d + 0.7 g DHA/d alone or in combination with home-based strength training on short physical performance battery (SPPB) test scores (Bischoff-Ferrari et al., 2020). Nonetheless, even if an effect of supplementation was present, it would have been difficult to observe in the latter study given that most participants had near-maximal SPPB scores at baseline. Several other factors have been posited to explain the inconsistency between studies in terms of the impact of LC n-3 PUFA supplementation on skeletal muscle outcomes (e.g., differences in sex, habitual protein intake, baseline health status, differences in outcome measures), and further research is needed to systematically test these hypotheses. Thus, it appears that the effect of LC n-3 PUFAs on skeletal muscle anabolism in older persons is mixed and unclear.
Endurance Exercise Training
In addition to the outer phospholipid membrane of skeletal muscle (sarcolemma), LC n-3 PUFA supplementation impacts the lipid composition of other key regulatory membranes in the cell, such as the mitochondria (Herbst et al., 2014). Mitochondrial membranes also exhibit different responses to LC n-3 PUFA supplementation compared with sarcolemmal membranes, with mitochondrial membranes appearing to be more susceptible to LC n-3 PUFA intake (Gerling et al., 2019). Given that oxidative phosphorylation, the process of adenosine triphosphate (ATP) production using adenosine diphosphate (ADP), inorganic phosphate (Pi), and oxygen, occurs in the mitochondria, the differential effect of LC n-3 PUFA intake on sarcolemmal and mitochondrial membranes could be physiologically relevant in the context of endurance exercise. In this regard, 12 weeks of 2 g/d EPA and 1 g/d DHA supplementation in young men increased ADP sensitivity (Herbst et al., 2014), whereas others have shown that LC n-3 PUFA supplementation can lower the oxygen cost of exercise (Hingley et al., 2017). Moreover, reports in rodents show that LC n-3 PUFA intake also reduces hindlimb oxygen consumption during contraction (Peoples & McLennan, 2010). Despite the potential for LC n-3 PUFAs to increase mitochondrial ADP sensitivity and decrease oxygen consumption during exercise, little evidence exists that LC n-3 PUFA ingestion translates to an ergogenic endurance exercise performance effect (Lewis et al., 2020).
Although existing evidence suggests that LC n-3 PUFA ingestion does not positively impact endurance exercise performance, it is important to note that nearly all studies in this field are conducted in younger adults (Heileson & Funderburk, 2020; Lewis et al., 2020) . This distinction is important because there may be fundamental differences in the physiology of younger and older persons that have a direct bearing on this area. For instance, it is not known if older persons require a greater relative dose of LC n-3 PUFAs to induce changes in cellular membranes compared with younger persons. Another, perhaps more compelling consideration is that LC n-3 PUFAs may only be ergogenic in the presence of dysfunction or suboptimal LC n-3 PUFA status. Indeed, mitochondrial dysfunction is often linked with impaired musculoskeletal health in older persons. In previous reports of enhanced muscle size with 6 months of LC n-3 PUFA ingestion in older persons (Smith et al., 2015) increases in mitochondrial transcripts were also identified (Yoshino et al., 2016). So while LC n-3 PUFA ingestion in younger persons may be ineffective in promoting endurance exercise performance it is possible that LC n-3 PUFA intake higher than population recommendations could be beneficial for master athletes.
LC n-3 PUFA STATUS
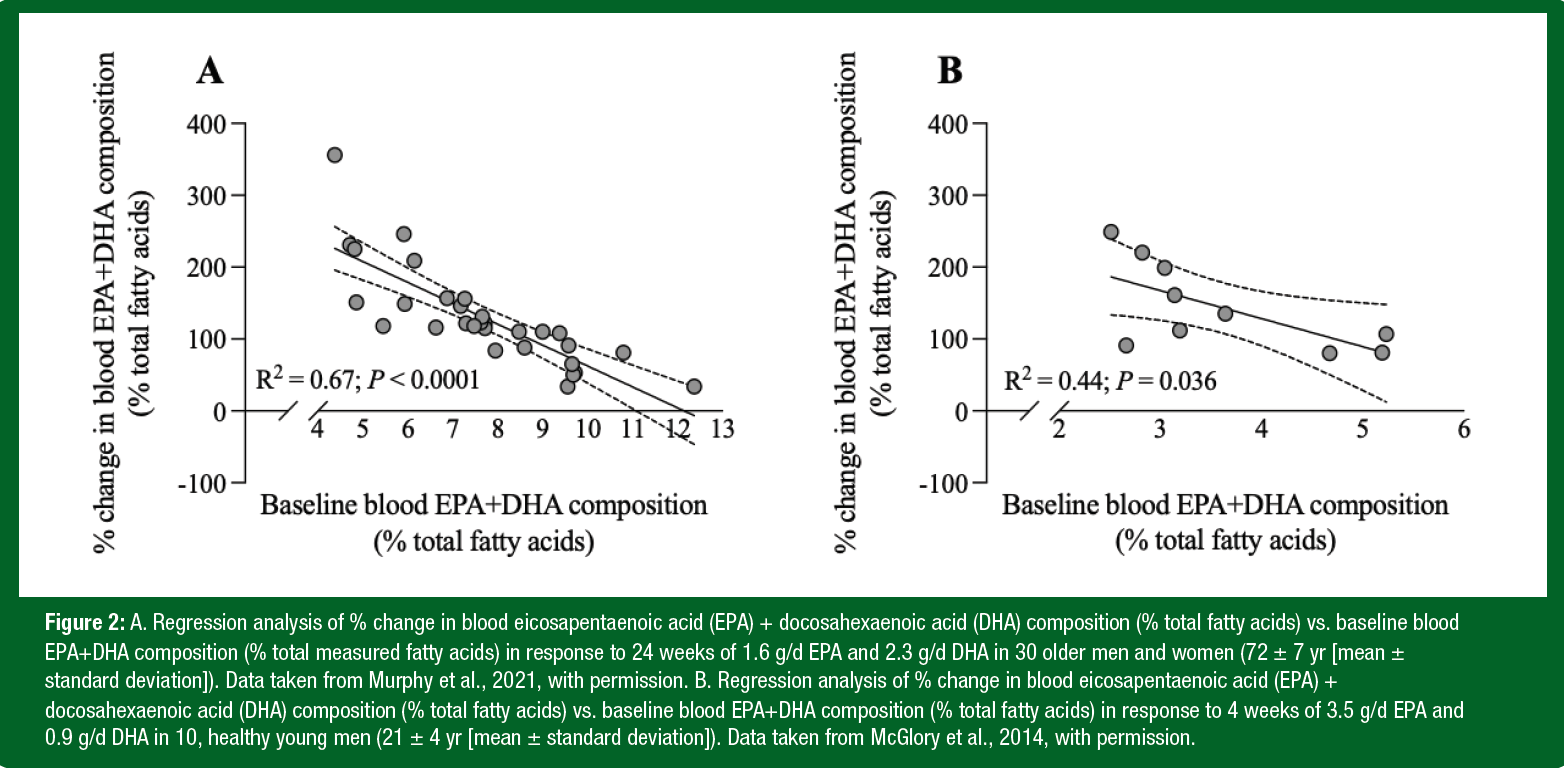
A common measure of LC n-3 PUFA status is known as the omega-3 index, which was developed to assess coronary heart disease risk largely from epidemiological data in individuals who already possessed cardiometabolic risk factors (Harris & Von Schacky, 2004). The omega-3 index is the EPA+DHA content of a red blood cell membrane as a percentage of the fatty acids contained in that membrane (von Schacky, 2015). Individuals with an omega-3 index of < 4% are considered high-risk, whereas those with an omega-3 index > 8% are considered low risk. Critically, it is known that ~25% of Canadian adults aged 60 to 79 years and ~50% of Canadian adults aged 20 to 39 years possess an omega-3 index < 4% (Demonty et al., 2021), with comparable numbers observed in the United States (Murphy et al., 2015). A recent cross-sectional study of 1,528 collegiate athletes identified a mean average omega-3 index of 4.3%, with no participants achieving the 8% mark (Ritz et al., 2020). Collectively, these findings would suggest that both younger and older adults would benefit from increased EPA+DHA ingestion (Demonty et al., 2021; Murphy et al., 2015; Ritz et al., 2020). In fact, a reanalysis of changes in EPA+DHA blood lipids in response to LC n-3 PUFA intake from our own work suggests a strong relationship between baseline LC n-3 PUFA status and the change in response to LC n-3 PUFA supplementation in both older (Murphy et al., 2021) and younger (McGlory et al., 2014) individuals (Figure 2). Put simply, those who have a lower baseline LC n-3 PUFA status prior to supplementation experience a greater increase post LC n-3 PUFA supplementation.
Although the omega-3 index is garnering significant interest, a number of issues should be considered when evaluating the relevance of the omega-3 index in the sport and exercise sciences. First, the relationship between the omega-3 index and exercise performance/recovery is far from conclusive with little data showing a positive correlation between the two factors. Second, the omega-3 index was developed predominantly in individuals who already possessed cardiometabolic risk factors, so the relevance to healthy athletes in the context of cardiovascular risk is questionable (Harris & Von Schacky, 2004). Third, changes in the LC n-3 PUFA composition of blood in response to LC n-3 PUFA supplementation do not necessarily reflect those of skeletal muscle, especially during the early (< 4 weeks) phase of supplementation (McGlory et al., 2014). This is because the changes in blood lipid profiles occur more rapidly following LC n-3 PUFA intake than do skeletal muscle (McGlory et al., 2014). Finally, the omega-3 index is a measure of the EPA+DHA content of a red blood cell membrane as a percentage of 26 measured fatty acids in that membrane (von Schacky, 2015). Thus, it is important to ensure that lipid analysis of blood samples from which the omega-3 index will be calculated is conducted by accredited laboratories or those with analytical standards capable of capturing all 26 fatty acids. Nevertheless, despite the aforementioned limitations the omega-3 index is a useful and practical biomarker of LC n-3 PUFA status in humans (Ritz et al., 2020) and future work that explores how changes in the omega-3 index relate to exercise performance and/or recovery are now warranted.
SOURCES OF LC n-3 PUFAs
Fish and Seafood
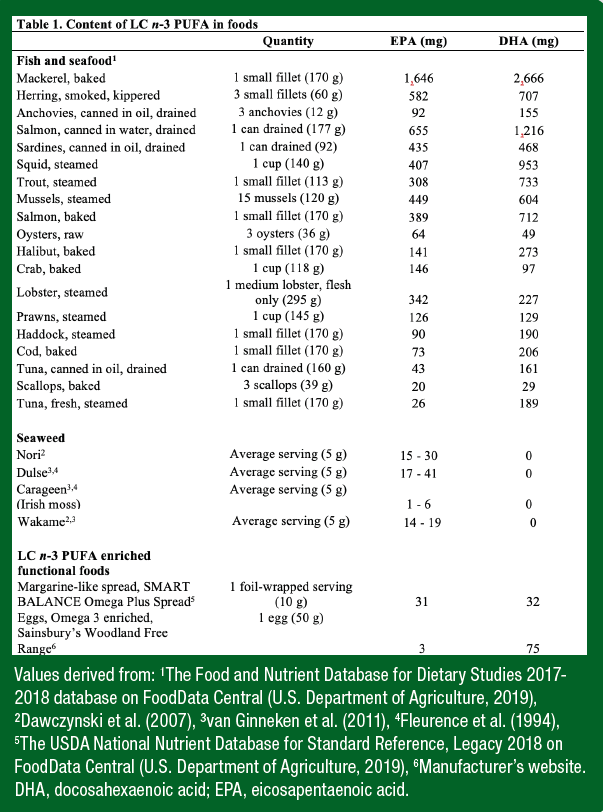
Although it is unclear if an ergogenic effect of LC n-3 PUFA intake on exercise performance exists, what is clear is that LC n-3 PUFA are an essential component of the human diet and many older adults possess low LC n-3 PUFA status (Demonty et al. 2021). Certain older persons may therefore benefit from increased LC n-3 PUFA intake. The principal source of LC n-3 PUFA in the diet is fish and seafood. Most fish contain EPA and DHA; however, the content varies substantially between species and is highest in oily fish such as salmon and mackerel, intermediate in shellfish such as oysters and mussels, and lower in white fish such as cod and haddock (Table 1). The EPA and DHA content also varies within a species depending on environmental variables (e.g., season, maturity and diet). Farmed fish are generally fed a mixture of vegetable and fish oils and tend to have a higher total lipid and n-6 PUFA content and a lower proportion of LC n-3 PUFA compared with that of wild fish. Together, these differences result in a similar quantity of LC n-3 PUFA per portion of farmed and wild fish (EFSA, 2005). The retention of EPA and DHA in fish during cooking is excellent (~85%) (Crowley & Gormley, 2018). Slightly more LC n-3 PUFA is lost as a result of canning (~24–49%); however, canned oily fish remains a good source. In addition to its LC n-3 PUFA content, further benefits of consuming fish include its content of the nutrients required for optimal health and athletic performance such as protein, iodine, selenium, calcium, and vitamins A, D, and B12.
Functional Foods
An increasing number of food products are enriched with LC n-3 PUFA (e.g., certain brands of eggs, peanut butter, juice, margarine, bread, yogurt, milk, soy beverages). These foods can be useful LC n-3 PUFA sources for individuals who avoid fish. The LC n-3 PUFA content of a food can be altered by adding EPA and DHA during the manufacturing process, or via feeding animals with short- or LC n-3 PUFA sources leading to the incorporation of the LC n-3 PUFA into eggs, dairy, and meat products. In general, eggs from chickens fed flax (rich in α-linolenic acid (ALA)) contain 60–100 mg DHA/egg, whereas eggs from chickens fed microalgae provide 100–150 mg DHA/egg. Enriched foods can vary considerably in their EPA and DHA content. Therefore, consumers are advised to check the label of these functional foods prior to purchase.
Dietary Supplements
Ideally, individuals should aim to meet their requirement for LC n-3 PUFA from the diet. However, LC n-3 PUFA supplements are useful in situations where this is not possible. LC n-3 PUFA supplements come in a variety of chemical forms (triglycerides, free fatty acids, ethyl esters, re-esterified triglycerides, and phospholipids) and are derived from a range of sources (fish oil, krill oil, cod liver oil, and algal oil). A typical fish oil supplement contains ~1,000 mg fish oil, providing 180 mg EPA and 120 mg DHA, but doses vary widely, ranging from ~150 to 1,300 mg EPA + DHA per capsule (Albert et al., 2015; Nichols et al., 2016). Some (Dyerberg et al., 2010), but not all (Hedengran et al., 2015), studies report higher bioavailability of LC n-3 PUFA provided as triglycerides or free fatty acids compared with ethyl esters. A recent study reported that triglyceride formulations produced a 1% greater increase in the omega-3 index compared with ethyl ester supplements at the same EPA+DHA dose, although both formulations were effective for improving LC n-3 PUFA status (Walker et al., 2019).
When choosing a supplement, a fish body oil supplement rather than a cod liver oil supplement is generally recommended, as the latter is high in vitamin A, which can lead to excessive intakes, particularly in at-risk populations like pregnant women. Vegans and vegetarians can choose a supplement containing microalgal oil, which typically provides around 100–300 mg DHA/capsule, with some supplements containing EPA as well. A further practical consideration is to consume LC n-3 PUFA supplements alongside a high fat meal to enhance absorption, particularly when the supplement is an ethyl ester formulation. It should also be noted that some studies have reported that a substantial proportion of LC n-3 PUFA supplements do not contain the amount of EPA+DHA advertised on the label and/or contain oxidized products exceeding industry-standard levels (Albert et al., 2015). Importantly, the impact of the oxidation of LC n-3 PUFA on their biological functions and health consequences remains to be established.
PRACTICAL APPLICATIONS
- Master athletes should consume LC n-3 PUFA in line with current population recommendations for health (250–500 mg/d). Although there is some evidence that greater intakes may enhance adaptations to resistance exercise, further research is needed before applied recommendations are possible.
- The omega-3 index is a useful measure of LC n-3 PUFA status; however, little evidence currently exists showing that it relates to exercise performance and/or recovery.
- Recommended LC n-3 PUFA intakes of 250–500 mg/d can be achieved via the consumption of 1 to 2 portions (100–140 g/portion) of oily fish (like mackerel, sardines, and salmon) per week.
- Functional foods enriched with LC n-3 PUFA generally contain substantially less LC n-3 PUFA per portion compared with that of fish but can be a valuable source among individuals who avoid fish.
- LC n-3 PUFA supplements are useful where adequate LC n-3 PUFA cannot be consumed via foods; however, there is some concern that certain supplements may contain oxidized products with potentially adverse health effects. Ideally choose a supplement that has undergone testing in an independent laboratory.
SUMMARY
In summary, the evidence to support an ergogenic effect of LC n-3 PUFA to enhance the adaptive response of skeletal muscle to resistance exercise training in older adults is mixed. The reasons underlying the mixed results is multifactorial and could be related to several methodological differences between studies such as LC n-3 PUFA dose, LC n-3 PUFA status of participants, and habitual protein intake. There is no evidence that LC n-3 PUFA intake enhances endurance exercise performance; however, no study has specifically studied the effect of LC n-3 PUFAs on endurance exercise performance in master athletes. Approximately 25% of older adults possess low LC n-3 PUFA status and thus older adults may benefit from increased LC n-3 PUFA either in the form of food or supplementation.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Albert, B.B., J.G. Derraik, D. Cameron-Smith, P.L. Hofman, S. Tumanov, S.G. Villas-Boas, and W.S. Cutfield (2015). Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 5:7928.
Anthony, R., M.J. Macartney, and G.E. Peoples (2021). The influence of long-chain omega-3 fatty acids on eccentric exercise-induced delayed muscle soreness: reported outcomes are compromised by study design issues. Int. J. Sport Nutr. Exerc. Metab. 31:143-153.
Bischoff-Ferrari, H.A., B. Vellas, R. Rizzoli, R.W. Kressig, J.A.P. da Silva, M. Blauth, and E.J. Orav (2020). Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. J. Am. Med. Assoc. 324:1855-1868.
Burke, L.M., J.A. Hawley, S.H. Wong, and A.E. Jeukendrup (2011). Carbohydrates for training and competition. J. Sports Sci. 29 (Suppl 1):S17-S27.
Crowley, L., and R. Gormley (2018). Omega-3 status of farmed salmon. SeaHealth-ucd(27). Retrieved from https://www.ucd.ie/foodandhealth/t4media/SeaHealth%2027b.pdf
Da Boit, M., R. Sibson, R., S. Sivasubramaniam, J.R. Meakin, C.A. Greig, R.M. Aspden, and S.R. Gray (2017). Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am. J. Clin. Nutr. 105:151-158.
Dawczynski, C., R. Schubert, and G. Jahreis (2007). Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 103:891–899.
Demonty, I., K. Langlois, L.S. Greene-Finestone, R. Zoka, and L. Nguyen (2021). Proportions of long-chain omega-3 fatty acids in erythrocyte membranes of Canadian adults: results from the Canadian Health Measures survey 2012-2015. Am. J. Clin. Nutr. 113:993-1008.
Dyerberg, J., P. Madsen, J.M. Møller, I. Aardestrup, and E.B. Schmidt (2010). Bioavailability of marine n-3 fatty acid formulations. Prostaglan. Leukot. Essent. Fatty Acids 83:137-141.
EFSA (2005). Opinion of the scientific panel on contaminants in the food chain on a request from the European parliament related to the safety assessment of wild and farmed fish. EFSA J. 236:1-118.
Fleurence, J., G. Gutbier, S. Mabeaul, and C. Leray (1994). Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 6:527-532.
Gerling, C.J., K. Mukai, A. Chabowski, G.J.F. Heigenhauser, G.P. Holloway, L.L. Spriet, and S. Jannas-Vela (2019). Incorporation of omega-3 fatty acids into human skeletal muscle sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front. Physiol. 10:348.
Gingras, A.A., P.J. White, P.Y. Chouinard, P. Julien, T.A. Davis, L. Dombrowski, and M.C. Thivierge (2007). Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 579:269-284.
Harris, W.S., and C. Von Schacky (2004). The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev. Med. 39:212-220.
Hedengran, A., P.B. Szecsi, J. Dyerberg, W.S. Harris, and S. Stender. (2015). n-3 PUFA esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: a randomized trial. Lipids 50:165-175.
Heileson, J.L., and L.K. Funderburk (2020). The effect of fish oil supplementation on the promotion and preservation of lean body mass, strength, and recovery from physiological stress in young, healthy adults: a systematic review. Nutr. Rev. 78:1001-1014.
Herbst, E.A., S. Paglialunga, C. Gerling, J. Whitfield, K. Mukai, A. Chabowski, and G.P. Holloway (2014). Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J. Physiol. 592:1341-1352.
Hingley, L., M.J. Macartney, M.A. Brown, P.L. McLennan, and G.E. Peoples (2017). DHA-rich fish oil increases the omega-3 index and lowers the oxygen cost of physiologically stressful cycling in trained individuals. Int. J. Sport Nutr. Exerc. Metab. 27:335-343.
Janssen, I. (2006). Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J. Am. Geriatr. Soc. 54:56-62.
Lewis, N.A., D. Daniels, P.C. Calder, L.M. Castell, and C.R. Pedlar (2020). Are there benefits from the use of fish oil supplements in athletes? A systematic review. Adv. Nutr. 11:1300-1314.
McGlory, C., S.D. Galloway, D.L. Hamilton, C. McClintock, L. Breen, J.R. Dick, and K.D. Tipton (2014). Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglan. Leukot. Essent. Fatty Acids 90:199-206.
McGlory, C., S. van Vliet, T. Stokes, B. Mittendorfer, and S.M. Phillips (2018). The impact of exercise and nutrition on the regulation of skeletal muscle mass. J. Physiol. 597:1251-1258.
McGlory, C., P.C. Calder, and E.A. Nunes (2019). The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 6:144.
McKendry, J., L. Breen, B.J. Shad, and C.A. Greig (2018). Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res. Rev. 45:62-82.
McKendry, J., B.J. Shad, B. Smeuninx, S.Y. Oikawa, G. Wallis, C. Greig, and L. Breen (2019). Comparable rates of integrated myofibrillar protein synthesis between endurance-trained master athletes and untrained older individuals. Front. Physiol. 10:1084.
McKendry, J., S. Joanisse, S. Baig, B. Liu, G. Parise, C.A. Greig, and L. Breen (2020). Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults. J. Gerontol. A Biol. Sci. Med. Sci. 75:1079-1088.
Murphy, C.H., E.M. Flanagan, G. De Vito, D. Susta, K.A.J. Mitchelson, E. de Marco Castro, J.M.G. Senden, J.P.B. Goessens, A. Mikłosz, A. Chabowski, R. Segurado, C.A. Corish, S.N. McCarthy, B. Egan, L.J.C. van Loon, and H.M. Roche (2021). Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n-3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 113:1411-1427.
Murphy, R.A., E.A. Yu, E.D. Ciappio, S. Mehta, and M.I. McBurney (2015). Suboptimal plasma long chain n-3 concentrations are common among adults in the United States, NHANES 2003-2004. Nutrients 7:10282-10289.
Nichols, P.D., L. Dogan, and A. Sinclair, (2016). Australian and New Zealand fish oil products in 2016 meet label omega-3 claims and are not oxidized. Nutrients 8:703.
Peoples, G.E, and P.L. McLennan (2010). Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br. J. Nutr. 104:1771-1779.
Ritz, P.P., M.B. Rogers, J.S. Zabinsky, V.E. Hedrick, J.A. Rockwell, E.G. Rimer, and M.S. Rockwell (2020). Dietary and biological assessment of the omega-3 status of collegiate athletes: a cross-sectional analysis. PLoS One 15:e0228834.
Rockwell, M., and P. Ritz (2021). Promoting optimal omega-3 fatty acid status in athletes. SSE #212.
Smith, G.I., P. Atherton, D.N. Reeds, B.S. Mohammed, D. Rankin, M.J. Rennie, and B. Mittendorfer (2011). Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 121:267-278.
Smith, G.I., S. Julliand, D.N. Reeds, D.R. Sinacore, S. Klein, and B. Mittendorfer (2015). Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 102:115-122.
The Scientific Advisory Committee on Nutrition (2004). The Scientific Advisory Committee on Nutrition and Committee on Toxicity advice on benefits and risks related to fish consumption. Retrieved from https://www.gov.uk/government/publications/sacn-advice-on-fish-consumption
U.S. Department of Agriculture (2019). FoodData Central. Available from U.S. Department of Agriculture, Agricultural Research Service FoodData Central Retrieved from fdc.nal.usda.gov.
US Department of Health and Human Services and US Department of Agriculture. (2015). 2015–2020 Dietary Guidelines for Americans. Retrieved from Washington, DC: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/
van Ginneken, V.J., J.P. Helsper, W. de Visser, H. van Keulen, and W.A. Brandenburg (2011). Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis. 10:104.
von Schacky, C. (2015). Omega-3 fatty acids in cardiovascular disease--an uphill battle. Prostaglan. Leukot. Essent, Fatty Acids 92:41-47.
Walker, R.E., K.H. Jackson, N.L. Tintle, G.C. Shearer, A. Bernasconi, S. Masson, and W.S. Harris (2019). Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 110:1034-1040.
Witard, O.C., and J.K. Davis. (2021). Omega-3 fatty acids for training adaptation and exercise recovery: a muscle-centric perspective in athletes. SSE #211.
Yoshino, J., G.I. Smith, S.C. Kelly, S. Julliand, D.N. Reeds, and B. Mittendorfer (2016). Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol. Rep. 4:e12785.







