Cold Water and Ice Slurry Ingestion for Reducing Body Temperature During Exercise in the Heat
Published
May 2018
Author
Ollie Jay and Nathan B. Morris, University of Sydney

KEY POINTS
- Cold water and ice slurry drinks are body-cooling methods that can be easily administered during exercise in the heat. However, core temperature is not reliably lowered compared to warmer drinks even during exercise at a fixed heat production.
- Cold fluid ingestion stimulates thermoreceptors in the abdomen that appear to decrease sweating independently of core and skin temperature.
- In dry and windy environments, the reduction in sweating with cold fluid ingestion decreases evaporative heat loss from the skin by a magnitude that at least negates the internal heat loss from warming the ingested cold fluid to body temperature.
- In humid and still environments, when sweat starts dripping from the skin, a net cooling effect from cold water and ice slurry ingestion during exercise should develop.
- The cooling benefits of cold fluid/ice ingestion during exercise are likely greatest for athletes with physiological disruptions to sweating, such as those with a spinal cord or burn injuries, as their capacity for skin surface evaporation is much lower.
- Cold fluid/ice ingestion during exercise is also more likely to be beneficial for lowering core temperature in athletes wearing protective equipment that impedes sweat evaporation (e.g., American football, ice hockey, motor sports, fencing).
- Cold water and ice slurry drinks are most effective for lowering athlete body temperature when administered either directly before exercise or during post-exercise recovery.
Introduction
During aerobic exercise, an enormous amount of metabolic energy is released as heat inside the body. This heat must be transported toward the skin and then dissipated to the surrounding environment to prevent large, and potentially dangerous, elevations in core body temperature (Kenny & Jay, 2013). From a sports perspective, large increases in body temperature also contribute to profound decrements in endurance performance, with time to exhaustion at a fixed intensity 50% shorter at an air temperature of 31°C compared to 11°C (Nybo et al., 2014). Cooling strategies that minimize the magnitude of exercise-induced hyperthermia are therefore keenly sought by athletes and sports practitioners alike.
Pre-cooling athletes, using cold water immersion (2-20°C), ice vests and/or neck cooling collars have the capacity to improve aerobic performance in the heat (Bongers et al., 2015). However, most of these strategies are not particularly feasible in low resource environments and can be problematic to apply (and replenish as needed) during exercise/competition. On the other hand, cold water or ice slurry (cold water mixed with crushed ice) ingestion before and/or during exercise offers a more convenient alternative. The efficacy of ice slurry or cold (< 10°C) water ingestion for improving aerobic performance in the heat has been comprehensively reviewed and the balance of evidence supports performance enhancement (Tan & Lee, 2015). What is less certain is whether cold water and/or ice slurry ingestion actually reduces the amount of heat stored inside the body. While cold drinks provide additional heat transfer (via conduction) inside the body, parallel modifications to sweating may under certain conditions reduce the amount of evaporative heat loss taking place at the skin surface. The main objectives of this sports science exchange article are to provide 1) an overview of the factors that influence body heat storage and ultimately the rise in core temperature, when cold water/ice slurry ingestion is given during exercise in the heat, and 2) provide practical considerations for when cold drinks should be recommended as an effective strategy for reducing body heat content during exercise.
HUMAN HEAT BALANCE AND BODY HEAT STORAGE
The amount of excess heat energy stored inside the body during exercise and/or heat exposure is determined by the fundamental laws of human heat balance:
Heat stored = Heat energy produced - Heat energy lost from skin surface
The heat energy produced (metabolic heat production: Hprod) emanates from inside the body and is a by-product of cellular metabolism. Hprod is the difference between metabolic energy expenditure (M) and the amount of external work (W) that is performed. It follows that M is determined by an athlete’s absolute rate of oxygen consumption (VO2; in Lmin-1) and the proportion of this oxygen that is used to catabolize carbohydrates relative to fats (i.e., respiratory quotient). The amount of external work performed is governed by the mechanical efficiency (percentage of metabolic energy that is used for external work) of the activity. During cycling, 20 to 25% of M is used for W, with the remaining 75 to 80% of M released as heat inside the body. On the other hand, running on a flat surface results in a negligible amount of external work (~0% of M) as the propulsion and breaking forces of gait result in equal amounts of positive and negative work, respectively (Margaria, 1968).
While a small amount of heat loss occurs via respiration, the vast majority of the heat energy lost from the body takes place at the skin surface, with heat exchange arising via four different avenues. Conduction (K), which is heat transfer through direct contact with a solid surface, is generally considered negligible for most exercise scenarios (Parsons, 2003). Convection (C), heat transfer between a solid and a moving fluid, is determined by 1) air flow across the skin surface, which can be either environmentally generated and/or self-generated air movement as an athlete propels themselves through an air mass; and 2) the temperature difference between the skin and the air. When air temperature exceeds skin temperature (~33 to 35°C) convective heat loss becomes convective heat gain. Radiation (R) is electromagnetic energy transfer between a relatively warm and cool body and is driven by the temperature difference between the skin and mean radiant temperature. The greatest source of thermal radiation in most sports-related settings is solar radiation, which is dependent on the time of day, season, cloud cover and latitude of location. Finally, evaporation (E) of sweat is the most important avenue of heat loss during exercise in the heat with 2,427 J of heat energy liberated for the evaporation of every 1 g of sweat (Parsons, 2003). The rate of evaporative heat loss is dependent on the difference in absolute humidity between the skin surface and surrounding environment, as well as the rate of airflow across the skin. The maximum rate of evaporation is also determined by the physiological capacity to saturate the skin surface with sweat. For example, even with maximal sweating a non-heat acclimated individual can only cover 85% of the skin surface with sweat whereas this value increases to 100% coverage with complete heat acclimation (Candas et al., 1979b). Evidently, athletes with injuries that reduce sweating capacity, e.g., spinal cord injured athletes or athletes with burn injuries, can only achieve much lower levels of sweat coverage and thus have a lower upper limit for evaporative heat loss under a fixed set of environmental conditions. Finally, as the skin becomes progressively wetter, sweating efficiency, i.e., the amount of sweat that evaporates relative to the amount of sweat produced, declines drastically (Candas et al., 1979a). Under conditions of reduced sweating efficiency, a reduction in sweating will have a smaller impact on evaporative heat loss.
The amount of heat energy stored inside the body (S) is determined by the cumulative difference between Hprod and net heat dissipation from skin to the surrounding environment (±K±C±R±E) (Parsons, 2003). During exercise at a fixed Hprod, the amount of evaporation (E), and therefore the amount of sweating, needed to limit S is progressively higher as air temperature increases. In a hot and humid environment, the amount of required evaporation may not be possible leading to an uncompensable heat stress scenario, which is characterised by a continually rising core (and usually skin) temperature due to the incessant accumulation of heat inside the body (Kenny & Jay, 2013). Under such conditions, if exercise continues the risk of heat-related illness and injury for an athlete is greatly elevated (Nybo et al., 2014). The observed rise in core temperature for a given amount of heat stored inside the body during exercise in the heat is predominantly determined by body mass (Kenny & Jay, 2013), with larger individuals at a fixed Hprod demonstrating much smaller rises in core temperature relative to their smaller counterparts, due to their larger heat sink (Dervis et al., 2016).
THE INFLUENCE OF COLD FLUID OR ICE SLURRY INGESTION ON HEAT BALANCE
The immediate benefit of the ingestion of a cold fluid or ice slurry is that it introduces an additional avenue of heat transfer (internal heat transfer) to the four avenues of heat transfer at the skin surface. Body heat storage is therefore determined by the cumulative difference between Hprod and the combined heat loss from the skin surface and any internal heat transfer with an ingested fluid. If skin surface heat loss remains unchanged following cold fluid or ice slurry ingestion, body heat storage will be lower, and the athlete will theoretically stay cooler. As skin surface heat loss during exercise in the heat primarily occurs via evaporation, any alteration in sweating will have the greatest impact on heat balance. Specifically, if skin surface evaporation is reduced by the same amount as the increase in internal heat transfer (inside the stomach), body heat storage will remain unaltered (Figure 1).
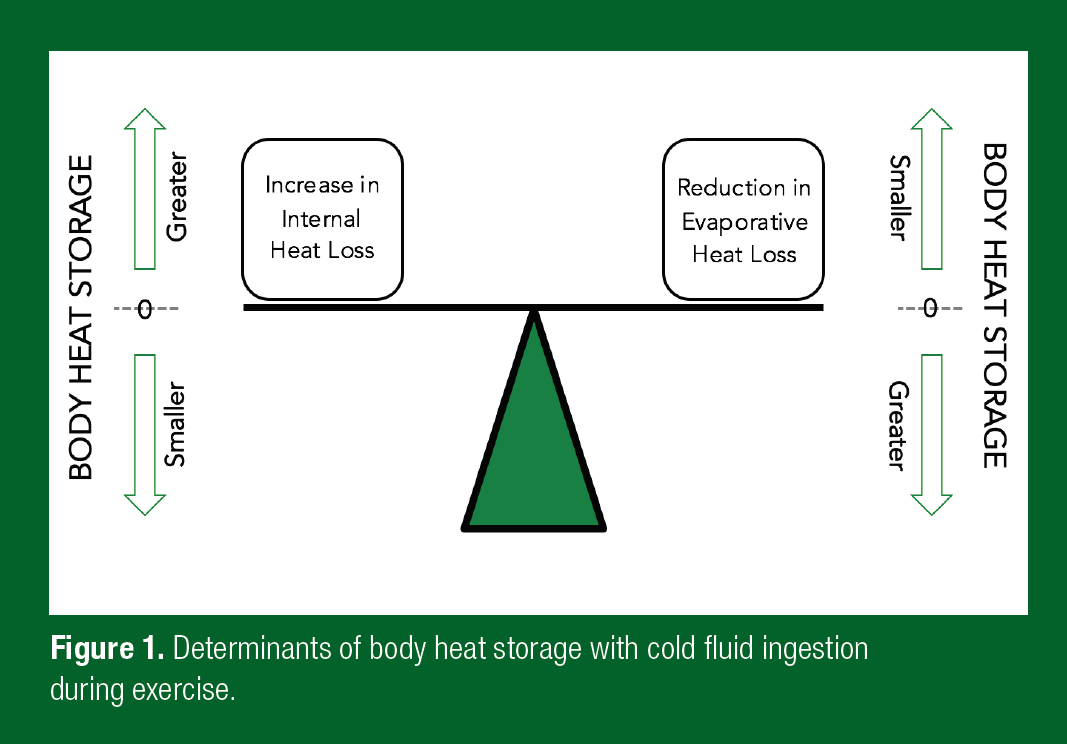
Sweating Responses with Cold Fluid or Ice Slurry Ingestion During Exercise and the Impact on Evaporation from the Skin
Sweating Responses with Cold Fluid or Ice Slurry Ingestion During Exercise and the Impact on Evaporation from the Skin
Decreases in sweating with cold water ingestion were reported as early as 1942 in a classic study by Pinson and Adolph (1942). More recently it has been shown that these alterations in sweating occur across the body within ~1 min of cold water (Morris et al., 2014) as well as ice slurry (Morris et al., 2016) ingestion during exercise (Figure 2). However, decreases in sweating are observed in advance of (and often without) any differences in core or skin temperatures, and are instead mediated by independent thermoreceptors most likely residing in, or around, the stomach (Morris et al., 2014).
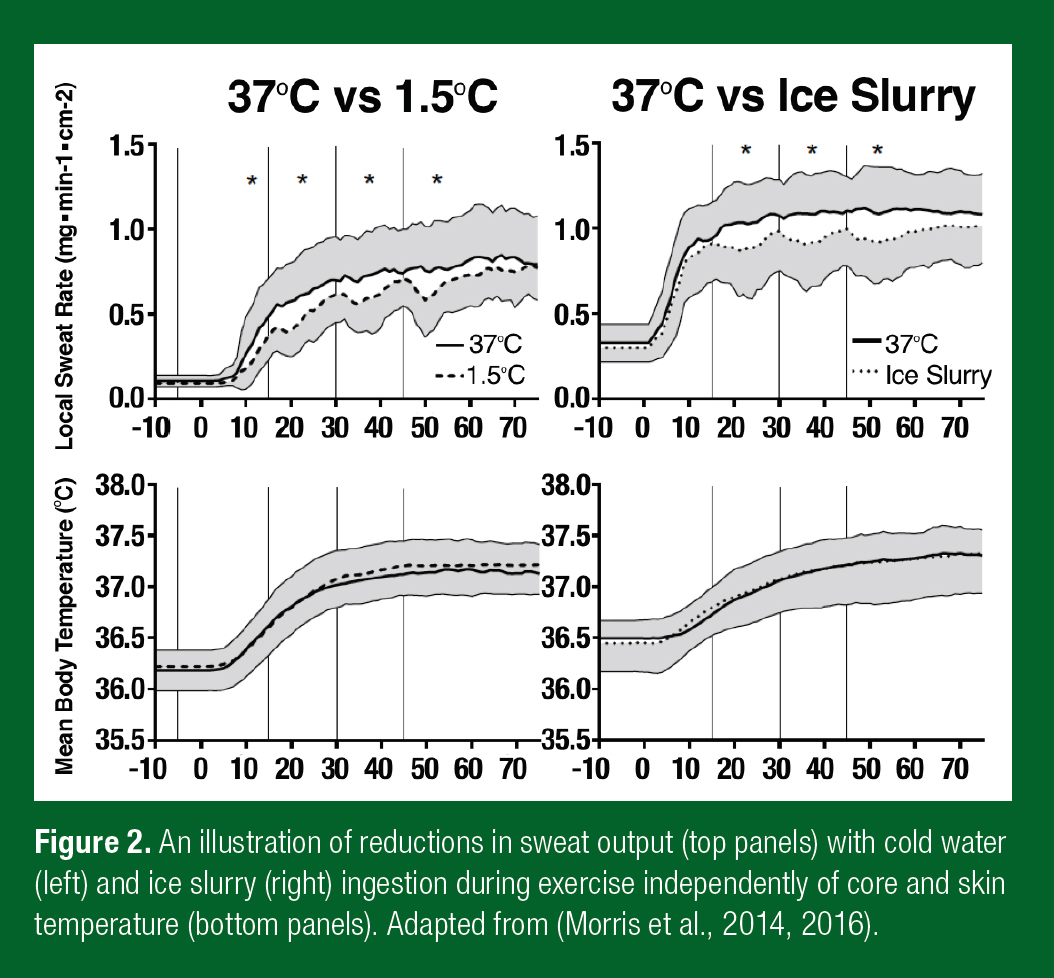
Given that the evaporation of 1 g of sweat yields 2,427 J of latent heat loss, the potential reduction in evaporative heat loss from the skin surface with cold water/ice slurry ingestion can be calculated. Moreover, this value can be compared to the parallel increase in internal heat loss estimated using the specific heat capacity of water (4.186 Jg-1) and the enthalpy of fusion of ice (334 Jg-1), as well as water mass/volume, and change in temperature required to equilibrate with body temperature.
Table 1 details captured studies from the literature that have reported whole-body sweat losses following the ingestion of cold water or ice and a warmer control fluid of a known volume during steady-state exercise in a non-encapsulated environment. Exercise performance studies were not included as self-selected exercise intensities (and therefore metabolic heat production) can be influenced by ingested fluid temperature, which would alter sweating independently of internal heat exchange (Mündel et al., 2006). Of the 10 studies included, seven tested cold fluid ingestion and three tested ice slurry ingestion. Also reported are the parallel differences in end-exercise core temperature between cold water or ice slurry ingestion and a warmer control fluid (Table 1). Twelve comparisons across the 10 studies were possible, with only five of 12 yielding a greater net heat loss with cold water/ice slurry ingestion compared to the control fluid (the increase in internal heat loss was greater than the decrease in heat skin surface evaporation). Irrespective of any disturbances in heat balance, a clear consistency among all captured studies is that at a fixed metabolic heat production, cold water/ice slurry ingestion during exercise does not lead to observable differences in the change in core temperature. In fact, only one study reported end-exercise values that were more than 0.1°C lower than with the ingestion of thermoneutral fluid at the same exercise intensity (Table 1). This observation is important as athletes exercising may feel cooler with cold fluid ingestion (Burdon et al., 2013), yet the data indicate that they will not actually be cooler.
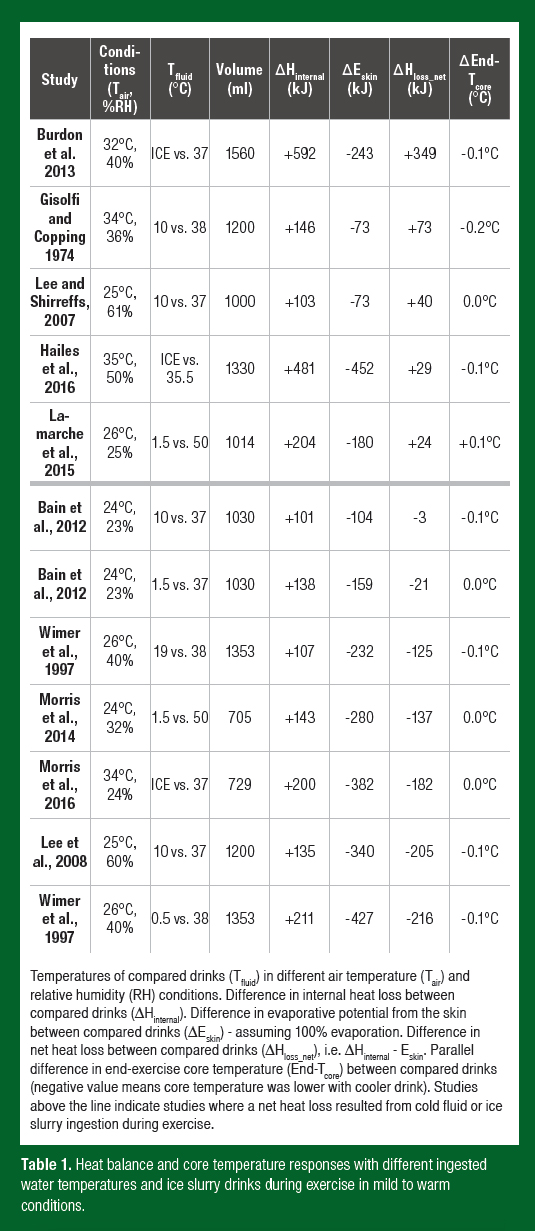
Are There Situations Where Cold Water/Ice Slurry Ingestion During Exercise Will Reduce Body Heat Storage?
The most important consideration for athletes and coaches using cold water or ice slurry ingestion during exercise as a cooling strategy is the prevailing environmental conditions that the athlete is exposed to. Most studies detailed in Table 1 were conducted in a relatively cool environment (i.e., air temperature ≤ 27°C), and it is noteworthy that in the three studies with the most hot/humid conditions (Burdon et al., 2013; Gisolfi & Copping, 1974; Hailes et al., 2016) ice slurry or cold water ingestion did seem to consistently result in slightly lower (0.1-0.2°C) end-exercise core temperatures.
The main advantage of the internal heat loss that occurs with cold water or ice slurry ingestion is that all heat transfer is 100% efficient. Whereas heat loss via the evaporation of sweat, as previously described, can be highly inefficient, particularly in hot, humid and still environments. Indeed, the evaporative efficiency of sweat in heat-acclimated elite athletes has been reported to be well below 50% (Malchaire et al., 2001). Therefore, under such conditions, reductions in skin surface sweating with cold water or ice slurry ingestion will not result in proportional reductions in evaporative heat loss, meaning that the increase in internal heat loss is more likely to exceed the reduction in evaporation leading to a greater likelihood of a lower body heat storage. It is clear, therefore, that the point at which cold water or ice slurry ingestion becomes beneficial in terms of reducing body heat storage is highly dependent on sufficient reductions in sweating efficiency. Decrements in sweating efficiency occur at different combinations of air temperature and humidity for a given metabolic heat production and rate of airflow across the skin; therefore, the boundary environmental conditions at which cold drinks are likely to reduce body heat storage will differ between sports.
As an example, the boundary combinations of air temperature and humidity for endurance cycling, a 50 km walking race and a marathon, at which cold water and ice slurry drinks are beneficial for eliciting a lower heat storage are illustrated in Figure 3.
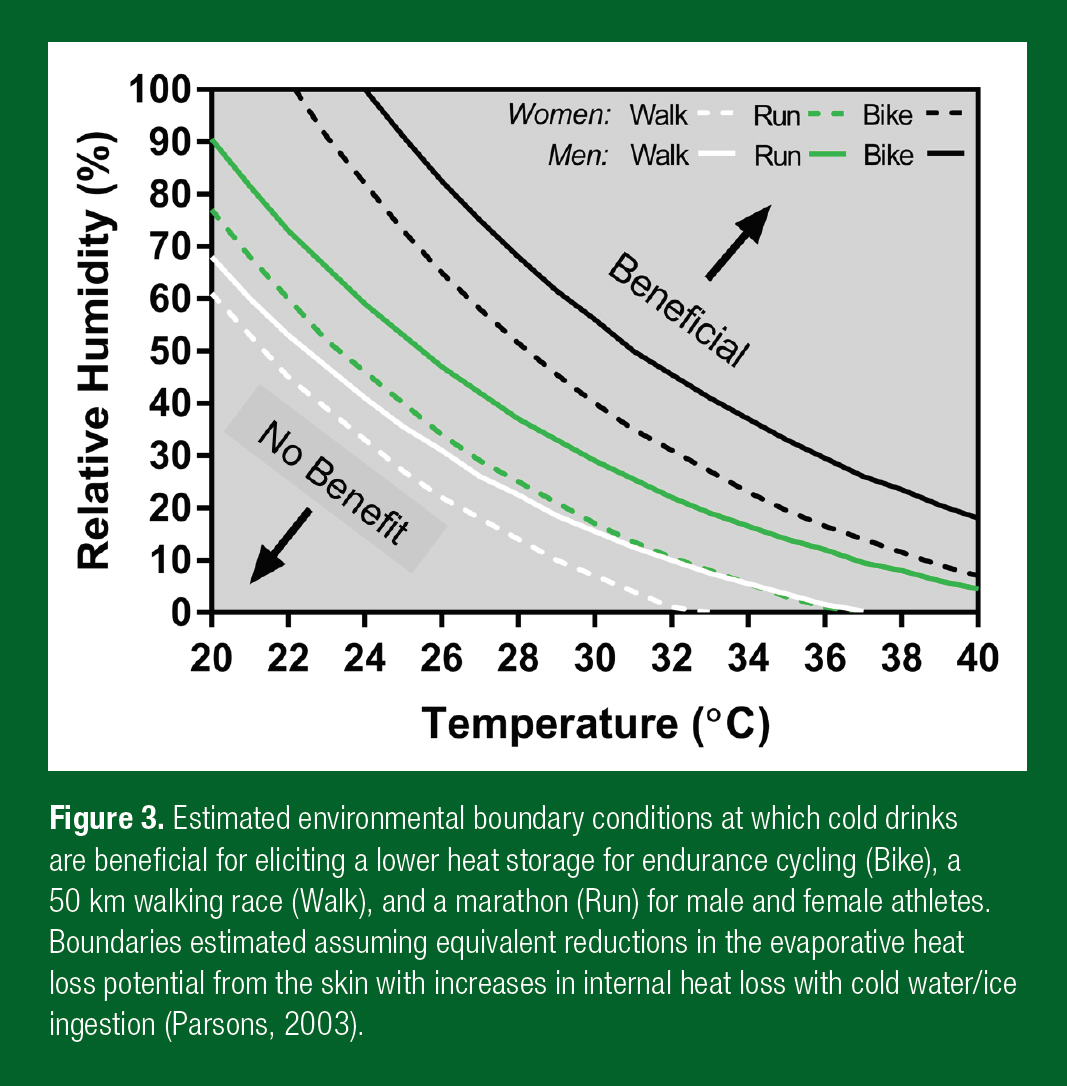
The relative humidity above which cold water and ice slurry ingestion will produce a net cooling effect becomes lower with increasing ambient air temperature for all activities. In fact, when high intensity exercise is coupled with a high air temperature and humidity, a reduction in sweating with cold water/ice ingestion does not impact evaporation at all because any additional sweat would only drip off the skin and maximum evaporation possible is achieved anyway. However, the boundary conditions change markedly between sporting events owing to the different self-generated airflows that the principal activities elicit. For example, airflow across the skin is much higher for a given metabolic heat production for a cyclist compared to a marathon runner, and even more so relative to a walker. A greater convective flow during cycling ultimately leads to a higher evaporative heat transfer coefficient and thus a greater drive for evaporation at the same vapour pressure gradient between the skin and air. The ambient humidity at which decrements in sweating efficiency occur with cycling is therefore greater than running, and greater for running than walking.
What About Cold Water/Ice Slurry Ingestion for Reducing Body Heat Storage Before or After Exercise?
Before exercise. In contrast to the studies assessing the cooling effect of drinking cold water/ice during exercise, it is evident that ingesting cold fluids before exercise is more effective for lowering body temperature. Since the cooling effect of cold drinks are negated by a reduction in skin surface sweating, it stands to reason that they are best ingested while minimal/no sweating is occurring. The existing literature shows that a decrease in core temperature of ~0.5°C consistently arises with cold water or ice slurry ingestion before exercise starts. An important consideration however is that pre-cooling with cold drinks should not cause core temperature to fall beyond the interthreshold zone (< 36.2°C) as shivering thermogenesis can be triggered. Also, a lower core temperature upon the commencement of exercise will most likely result in a delayed onset of sweating, and possibly a blunted vasodilatory response, likely leading to greater rates of heat storage and core temperature rise during the early stages of exercise, as has been demonstrated with other forms of pre-cooling (Lee & Haymes, 1995). Nevertheless, the ingestion of cold drinks before exercise appears to reliably extend exercise duration required, at a fixed metabolic heat production, to reach a critical absolute core temperature threshold (Tan & Lee, 2015).
After exercise. While cold fluid/ice drinks have the potential to elicit a cooling effect during exercise under certain conditions, the relative benefit of drinking cold fluids during post-exercise recovery may be greater. It is well documented that a rapid decline in sweating (~50% reduction within 5 min) occurs as soon as exercise stops due to the influence of non-thermal factors (e.g., baroreceptor input) (Kenny & Jay, 2013) . Therefore, reducing skin surface sweating due to the stimulation of abdominal thermoreceptors from drinking cold fluids or ice slurries after exercise would have less of an impact on body heat storage because sweat output rapidly declines anyway. Cold drinks may therefore be particularly effective for athletes competing in intermittent sports with regular breaks, e.g., tennis or tournament-style events featuring multiple games/matches in one day. Only a small number of studies have examined the efficacy of cold fluid ingestion during the post-exercise period but Stanley et al. (2010) reported a ~0.4°C lower rectal temperature 50 min post-exercise with ice slurry ingestion relative to cold water. Lee et al. (2013) also reported a ~0.2 - 0.3°C greater decline in core temperature with post-exercise ingestion of 4°C water compared to 28°C water.
OTHER CONSIDERATIONS
Athletes that are either fully (e.g., fencers, motorsport drivers/riders) or partially (e.g., American football and ice hockey players) encapsulated by protective equipment/clothing are unable to fully modify skin surface heat loss via changes in sweating, due to the fact that sweat evaporation is blunted by the properties of the clothing system. Therefore, cold fluid ingestion by these individuals should create a large net cooling effect since reductions in sweating will not greatly influence skin surface evaporation, yet internal heat exchange with a cold ingested beverage with remain 100% efficient. However, future studies are required to experimentally demonstrate this notion. Similarly, athletes with a physiological disruption to sweating owing to injuries to the spinal cord or skin surface (e.g., burns) will also likely benefit from cold fluid/ice ingestion. The maximum proportion of the skin surface that these individuals can physiologically saturate with sweat, and the accompanying level of evaporative heat loss, is drastically lower relative to their non-injured counterparts (Crandall & Davis, 2010). Therefore, any reductions in sweating with cold fluid ingestion will likely have a smaller impact on net heat loss. It is also possible, but not yet determined, that sweating remains uninterrupted by the stimulation of abdominal thermoreceptors with cold fluid ingestion in some athletes with a thoracic or cervical spinal cord injury.
Practical Applications
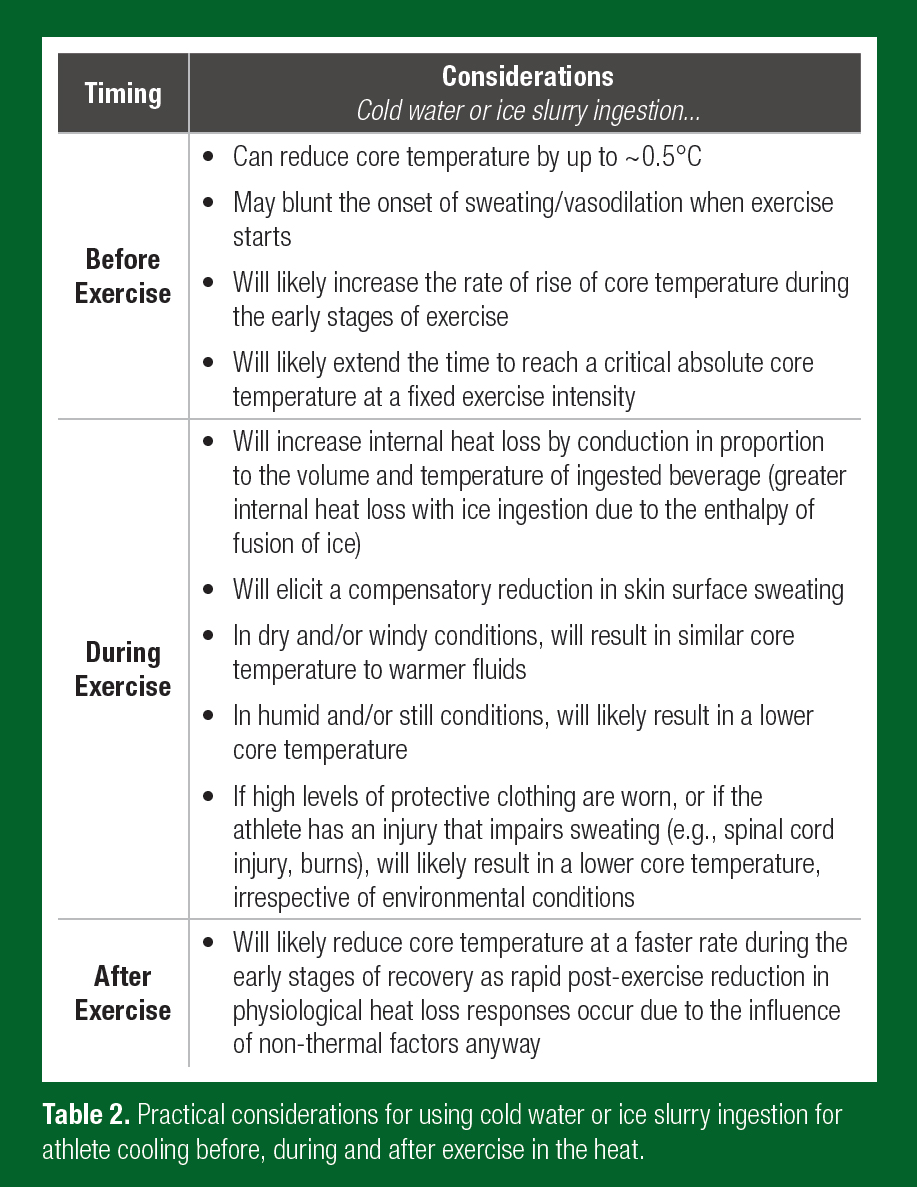
A summary table detailing the key practical points associated with the use of cold water or ice slurry ingestion as a cooling strategy for reducing body heat storage, before exercise, during exercise and after exercise, is provided in Table 2.
Summary
While cold water and ice slurry ingestion during exercise seems to improve endurance performance in the heat and may help an athlete feel cooler, it does not necessarily result in a net cooling effect due to a compensatory reduction in sweating and the potential for evaporative heat loss at the skin surface. However, in hot, humid and/or still environments, the reductions in sweating efficiency (i.e., the amount of sweat that evaporates) ensures that cold water/ice ingestion will become more beneficial for cooling, as internal heat exchange with an ingested fluid remains 100% efficient irrespective of the external climate. Therefore, cold water and ice slurry ingestion during exercise can be recommended for cooling an athlete when it is hot, humid and still; but not in warm, dry and windy environments. Cold water and ice slurry ingestion is probably most effective for cooling when administered prior to exercise – before the athlete begins to sweat, and/or after exercise has stopped – when sweat rates begin to decline. On the whole, cold water/ice ingestion is probably most beneficial for cooling walkers and runners rather than cyclists due to the large differences in airflow across the skin, which greatly alters the evaporative efficiency of sweat. Athletes with physiological impairments of sweating, such as spinal cord injured athletes, as well as athletes wearing equipment/clothing that present a high level of evaporative resistance (e.g., racing car drivers, American football players) are also more likely to benefit most from the cooling effects of cold water or ice slurry ingestion.
REFERENCES
Bain, A.R., N.C. Lesperance, and O. Jay (2012). Body heat storage during physical activity is lower with hot fluid ingestion under conditions that permit full evaporation. Acta Physiol. 206:98–108.
Bongers, C.C.W.G., D.H.J. Thijssen, M.T.W. Veltmeijer, M.T.E. Hopman, and T. M. Eijsvogels (2015). Precooling and percooling (cooling during exercise) both improve performance in the heat: a meta-analytical review. Br. J. Sports Med. 49:377–384.
Burdon, C.A., M.W. Hoon, N.A. Johnson, P.G. Chapman, and H.T. O’Connor (2013). The effect of ice slushy ingestion and mouthwash on thermoregulation and endurance performance in the heat. Int. J. Sport Nutr. Exerc. Metab. 23:458–469.
Candas, V., J.P. Libert, and J.J. Vogt (1979a). Human skin wettedness and evaporative efficiency of sweating. J. Appl. Physiol. 46:522–528.
Candas, V., J.P. Libert, and J.J. Vogt (1979b). Influence of air velocity and heat acclimation on human skin wettedness and sweating efficiency. J. Appl. Physiol. 47:1194–1200.
Crandall, C.G., and S.L. Davis (2010) Cutaneous and sudomotor responses in human skin grafts. J. Appl. Physiol. 109:1524-30.
Dervis, S., G.B. Coombs, G.K. Chaseling, D. Filingeri, J. Smoljanic, and O. Jay (2016). A comparison of thermoregulatory responses to exercise between mass-matched groups with large differences in body fat. J. Appl. Physiol.120:615–623.
Gisolfi, C.V., and J.R. Copping (1974). Thermal effects of prolonged treadmill exercise in the heat. Med. Sci. Sports 6:108–113.
Hailes, W.S., J.S. Cuddy, K. Cochrane, and B.C. Ruby (2016). Thermoregulation during extended exercise in the heat: Comparisons of fluid volume and temperature. Wilderness Environ. Med. 27:386–392.
Kenny, G.P., and O. Jay (2013). Thermometry, calorimetry, and mean body temperature during heat stress. Compr. Physiol. 3:1689–1719.
Lamarche, D.T., R.D. Meade, R. McGinn, M.P. Poirier, B.J. Friesen, and G.P. Kenny (2015). Temperature of ingested water during exercise does not affect body heat storage. Med. Sci. Sports Exerc. 47:1272–1280.
Lee, D.T., and E.M. Haymes (1995). Exercise duration and thermoregulatory responses after whole body precooling. J. Appl. Physiol. 79:1971–1976.
Lee, J.K.W., and S.M. Shirreffs (2007). The influence of drink temperature on thermoregulatory responses during prolonged exercise in a moderate environment. J. Sports Sci. 25:975–985.
Lee, J.K.W., R.J. Maughan, and S.M. Shirreffs (2008). The influence of serial feeding of drinks at different temperatures on thermoregulatory responses during cycling. J. Sports Sci. 26:583–590.
Lee, J.K.W., Z.W. Yeo, A.Q.X. Nio, A.C.H. Koh, Y.S. Teo, L.F. Goh, P.M. Tan, and C. Byrne (2013). Cold drink attenuates heat strain during work-rest cycles. Int. J. Sports Med. 34:1037–1042.
Malchaire, J., A. Piette, B. Kampmann, P. Mehnert, H. Gebhardt, G. Havenith, E, Den Hartog, I. Holmer, K. Parsons, G. Alfano, B. Griefahn (2001). Development and validation of the predicted heat strain model. Ann. Occup. Hyg. 45:123–135.
Margaria, R. (1968). Positive and negative work performances and their efficiencies in human locomotion. Int. Z. Für Angew. Physiol. Einschließlich Arbeitsphysiologie 25:339–351.
Morris, N.B., A.R. Bain, M.N. Cramer, and O. Jay (2014). Evidence that transient changes in sudomotor output with cold and warm fluid ingestion are independently modulated by abdominal, but not oral thermoreceptors. J. Appl. Physiol. 116:1088–1095.
Morris, N.B., G. Coombs, and O. Jay. (2016). Ice Slurry Ingestion Leads to a Lower Net Heat Loss during Exercise in the Heat. Med. Sci. Sports Exer. 48:114–122.
Mündel, T., J. King, E. Collacott, and D.A. Jones (2006). Drink temperature influences fluid intake and endurance capacity in men during exercise in a hot, dry environment. Exp. Physiol. 91:925–933.
Nybo, L., P. Rasmussen, and M.N. Sawka (2014). Performance in the heat-physiological factors of importance for hyperthermia-induced fatigue. Compr. Physiol. 4:657–689.
Parsons, K. (2003). Human Thermal Environments (Second Ed.). New York, NY: Taylor & Francis Inc.
Pinson, E.A., and E.F. Adolph (1942). Heat exchanges during recovery from experimental deficit of body heat. Am. J. Physiol. 136:105–114.
Stanley, J., M. Leveritt, and J.M. Peake (2010). Thermoregulatory responses to ice-slush beverage ingestion and exercise in the heat. Eur. J. Appl. Physiol. 110:1163–1173.
Tan, P.M.S., and J.K.W. Lee. (2015). The role of fluid temperature and form on endurance performance in the heat. Scand. J. Med. Sci. Sports 25:39–51.
Wimer, G.S., D.R. Lamb, W.M. Sherman, and S.C. Swanson (1997). Temperature of ingested water and thermoregulation during moderate-intensity exercise. Can. J. Appl. Physiol. 22:479–493.








