Plant versus animal-based proteins to support muscle conditioning
KEY POINTS
- The increase in muscle protein synthesis rates following ingestion of a single, meal-sized amount (20-25 g) of plant-derived protein seems to be less robust when compared with the response following ingestion of an equivalent amount of animal-derived protein.
- The proposed lesser anabolic properties of plant-derived proteins are considered to be attributed to a lower essential amino acid content when compared with high-quality animal-derived proteins, with many plant-derived proteins being deficient in one or more specific amino acids, such as lysine or methionine.
- There is considerable variation in the protein content of various plant-based protein sources and the amino acid composition of their derived proteins.
- The increase in muscle protein synthesis rates following ingestion of a plant-based protein source or a plant-derived protein isolate or concentrate may be improved by increasing the amount of protein ingested, by consuming a combination of different plant-based protein sources or plant-derived proteins, and/or by fortifying these proteins with the deficient (free) amino acid(s).
- Consumption of a defined amount of protein in the form of plant-based whole foods generally requires a greater quantity of food, in both weight and caloric content, to be consumed when compared with the consumption of an equivalent amount of protein in the form of a high-quality animal-based protein source.
- Muscle conditioning in athletes does not need to be compromised when adopting a plant-based diet as long as sufficient protein is consumed from a large variety of different plant-based protein sources.
INTRODUCTION
Protein ingestion stimulates skeletal muscle protein synthesis (Groen et al., 2015). Muscle protein synthesis rates are further increased when protein is ingested during recovery from exercise, thereby facilitating muscle conditioning (Moore et al., 2009b). Ingestion of 20 g of a high-quality protein can maximize muscle protein synthesis rates for several hours (Churchward-Venne et al., 2020; Moore et al., 2009a; Witard et al., 2014). The muscle protein synthetic response to feeding is largely determined by the amount of protein ingested (Churchward-Venne et al., 2020; Moore et al., 2009a; Robinson et al., 2013; Witard et al., 2014), the protein digestion and absorption kinetics (Boirie et al., 1997), and the amino acid composition of the protein (source) that is consumed (Tang et al., 2009; Yang et al., 2012b). The muscle protein synthetic response to protein ingestion can, therefore, vary substantially between different dietary protein sources (Gorissen et al., 2016; Tang et al., 2009; Yang et al., 2012b). The differential muscle protein synthetic response to feeding largely depends on the post-prandial rise in circulating essential amino acid concentrations, with plasma leucine concentration being of particular importance (Dickinson et al., 2014; Koopman et al., 2005; Rieu et al., 2006; Wall et al., 2013; Wilkinson et al., 2013). The post-prandial rise in circulating amino acids and the subsequent increase in muscle protein synthesis rate are regulated on various levels, ranging from protein digestion and amino acid absorption, splanchnic amino acid extraction, post-prandial tissue perfusion, amino acid uptake in muscle, activation of the muscle protein synthetic machinery, and finally muscle protein synthesis (Groen et al., 2015; Trommelen et al., 2021a). To date, most studies have assessed muscle protein synthesis rates following dairy protein (Churchward-Venne et al., 2019a; Pennings et al., 2011; Tang et al., 2007; Tipton et al., 2004; Wilkinson et al., 2018) and meat (Burd et al., 2015; Robinson et al., 2013) ingestion.
With the world population estimated to reach nearly 10 billion by 2050, it may no longer be feasible to produce sufficient amounts of conventional animal-based, protein-dense foods to meet the increasing global dietary protein demands. This has sparked the trend of transitioning toward consuming a (more) plant-based diet. Changing to a (more) plant-based diet will increase the consumption of plant-based proteins at the expense of animal-based proteins. Although the current market already offers a wide selection of plant-based protein sources and plant-derived protein concentrates or isolates, there are only few studies that have assessed the bioavailability and anabolic properties of plant-based proteins in vivo in humans (Churchward-Venne et al., 2019b; Gorissen et al., 2016; Pinckaers et al., 2021; Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012a). These studies tend to show that ingestion of plant-derived proteins, such as soy and wheat protein, do not have the same capacity to increase muscle protein synthesis rates when compared with the ingestion of an equivalent amount of animal-derived protein (Gorissen et al., 2016; Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012a). Consequently, concerns have been raised as to whether the consumption of a (more) plant-based diet and plant-derived proteins at the expense of animal-based proteins could compromise muscle protein synthesis and, as such, negatively impact muscle health and performance.
PROTEIN BIOAVAILABILITY
After food ingestion, dietary protein undergoes mechanical and chemical breakdown into smaller constituents in the mouth, stomach, and small intestine (Trommelen et al., 2021b) after which the amino acids can be absorbed in the intestinal lumen. A substantial part of the absorbed amino acids will be retained and metabolized in the splanchnic region, but most protein-derived amino acids will be released into the circulation. The quantitative assessment of protein digestibility, amino acid absorbability, splanchnic extraction, and amino acid release in the circulation is complex, and only a few studies have tried to quantify post-prandial protein handling in vivo in humans (Groen et al., 2015). In general, proteins in plant-based whole foods are not as effectively absorbed when compared with proteins in animal-based whole foods (Kashyap et al., 2018; 2019). The lower absorbability of plant-based proteins can be largely attributed to anti-nutritional factors present in plant-based protein sources (Sarwar Gilani et al., 2012). When a plant-based protein is extracted from the protein source and purified from anti-nutritional factors to produce a plant-derived protein isolate or concentrate, the subsequent protein absorbability typically reaches levels similar to those observed for conventional animal-based protein sources (Gausserès et al., 1997). This implies that the lower absorbability of plant-based protein sources is not a characteristic of the intrinsic protein per se but simply a result of the whole food matrix of the protein source.
Besides the overall bioavailability of a protein, there are ample data to suggest that the rate of amino acid absorption forms an independent factor that modulates the muscle protein synthetic response to feeding (Gorissen et al., 2020; Koopman et al., 2009b; Pennings et al., 2011). There are few data available on the amino acid absorption kinetics following the ingestion of plant-based protein sources or plant-derived protein isolates or concentrates. In contrast to many plant-based protein sources, ingestion of plant-derived protein isolates or concentrates is generally followed by rapid increases in plasma amino acid concentrations that do not differ substantially from most animal-derived proteins or protein sources (Brennan et al., 2019; Gorissen et al., 2016; Liu et al., 2019; Pinckaers et al., 2021; Tang et al., 2009). It is more than likely that the anti-nutritional factors present in plant-based whole foods not only compromise protein bioavailability, but also attenuate the post-prandial rise in circulating plasma amino acid concentrations. Because of the apparent differences in protein absorbability, protein digestion, and amino acid absorption kinetics, we need to be careful when referring to plant-based proteins as either plant-based protein sources (whole foods) or as plant-derived protein isolates or concentrates.
PROTEIN QUALITY
The increase in plasma amino acid concentrations following protein ingestion activates the protein synthetic machinery in skeletal muscle tissue and also provides the necessary building blocks required to allow muscle protein synthesis rates to increase. The essential amino acids are considered to be mainly responsible for the stimulation of muscle protein synthesis. Consequently, proteins with high(er) essential amino acid contents are considered high(er) quality proteins and are more likely to (strongly) stimulate muscle protein synthesis. The essential amino acid content of plant-derived proteins are generally lower when compared with animal-derived proteins (Gorissen et al., 2018; van Vliet et al., 2015). However, there are also plant-derived proteins (such as: soy, brown rice, canola, pea, corn, and potato protein) that have relatively high essential amino acid content, meeting the requirements recommended by the WHO/FAO/UNU (FAO/WHO/UNU expert consultation, 2007). Therefore, various plant-based proteins may provide sufficient essential amino acids to allow a strong increase in muscle protein synthesis. From all amino acids, leucine seems to possess the strongest anabolic properties. The leucine content of a dietary protein is, therefore, considered another key characteristic that determines the anabolic properties of a protein. The current leucine requirement within a given protein source is set at 5.9% by the WHO/FAO/UNU (FAO/WHO/UNU expert consultation, 2007). Whereas plant-based proteins like hemp (5.1% leucine) and lupin (5.2%) fall short, other proteins like soy (6.9%), canola (6.9%), pea (7.2%), brown rice (7.4%), potato (8.3%), and corn (13.5%) protein contain leucine in amounts that exceed the recommended requirements. The leucine content of potato protein (8.3%) is even higher when compared with casein (8.0%) or egg (7.0%) protein. Furthermore, the leucine content of corn protein (13.5%) is higher than whey protein (11.0%), with the latter being regarded the protein with the highest leucine content and the strongest anabolic potential among the conventional animal-derived proteins. Besides the relatively low essential amino acid content (i.e., low leucine content), many plant-based proteins are deficient in one or more specific amino acid. Plant-based proteins are often particularly low in lysine and/or methionine content (ranging from 1.4% to 6.0% and 0.2% to 2.5%, respectively) when compared with animal-based proteins (ranging from 5.3% to 9.0% and 2.2% to 2.8%, respectively). However, there is considerable variability in amino acid composition between the many different plant-based protein sources and plant-derived proteins (Gorissen et al., 2018; van Vliet et al., 2015).
Only a handful of studies have directly compared post-prandial muscle protein synthesis rates following ingestion of plant- versus animal-derived proteins (Churchward-Venne et al., 2019b; Gorissen et al., 2016; Pinckaers et al., 2021; Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012b). Ingestion of soy protein has been shown to be less effective in stimulating muscle protein synthesis rates when compared with an equivalent amount of whey protein at rest and during recovery from exercise (Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012b). Furthermore, Yang et al. (2012b) showed that ingesting a greater amount (40 g vs. 20 g) of soy protein did not compensate for the lesser muscle protein synthetic response when compared with the ingestion of 20 g whey protein, whereas no significant increase in muscle protein synthesis rate was observed following the ingestion of 35 g wheat protein in a group of older men (Gorissen et al., 2016). However, a robust increase in muscle protein synthesis rate was observed when the amount of wheat protein was increased to 60 g, providing the equivalent amount of leucine that was provided in 35 g whey protein (Gorissen et al., 2016). These data support the hypothesis that differences in amino acid composition can be, at least in part, compensated for by ingesting larger amounts of a certain protein or protein source.
IMPROVING PROTEIN FUNCTIONALITY
The lower anabolic properties of plant-based proteins compared with animal-based proteins may be attributed to differences in protein absorbability, protein digestion and amino acid absorption kinetics, and/or amino acid composition of the proteins. Depending on the factor(s) responsible, there are various strategies that can be applied to augment the anabolic properties of plant-based proteins. Processing of whole foods can strongly increase the absorbability of the intrinsic protein (Devi et al., 2018). Extraction of protein and purification from anti-nutritional factors will improve the bioavailability of the protein (Gausserès et al., 1997). Heat treatment and hydrolysation of the protein may further increase digestibility and/or improve protein digestion and amino acid absorption kinetics (Gorissen et al., 2015; Koopman et al., 2009a). Such processing is typically applied in most plant- as well as animal-based protein sources that we purchase either as (processed) food products or as protein isolates or concentrates.
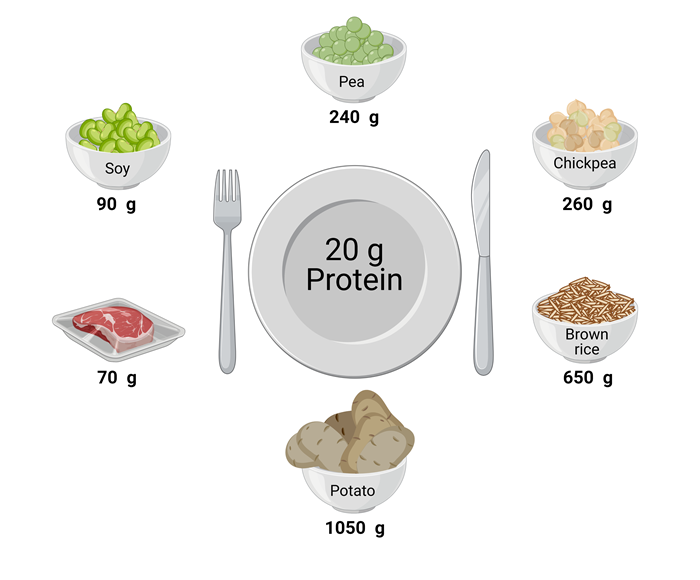
The lesser anabolic properties of some plant-based proteins may also be attributed to the low(er) essential amino acid content and/or due to specific amino acid deficiencies. A simple way to compensate for the lower protein quality of a plant-based protein source is to consume more (Yang et al., 2012a). Though such a strategy is easy to apply when consuming a plant-derived protein isolate or concentrate, it may not be practical or feasible when consuming plant-based whole foods. Due to the low protein density of most plant-based protein sources, ingesting a greater amount of protein would translate into a disproportionate increase in the volume and caloric content of the plant-based food that would need to be consumed (Figures 1, 2).
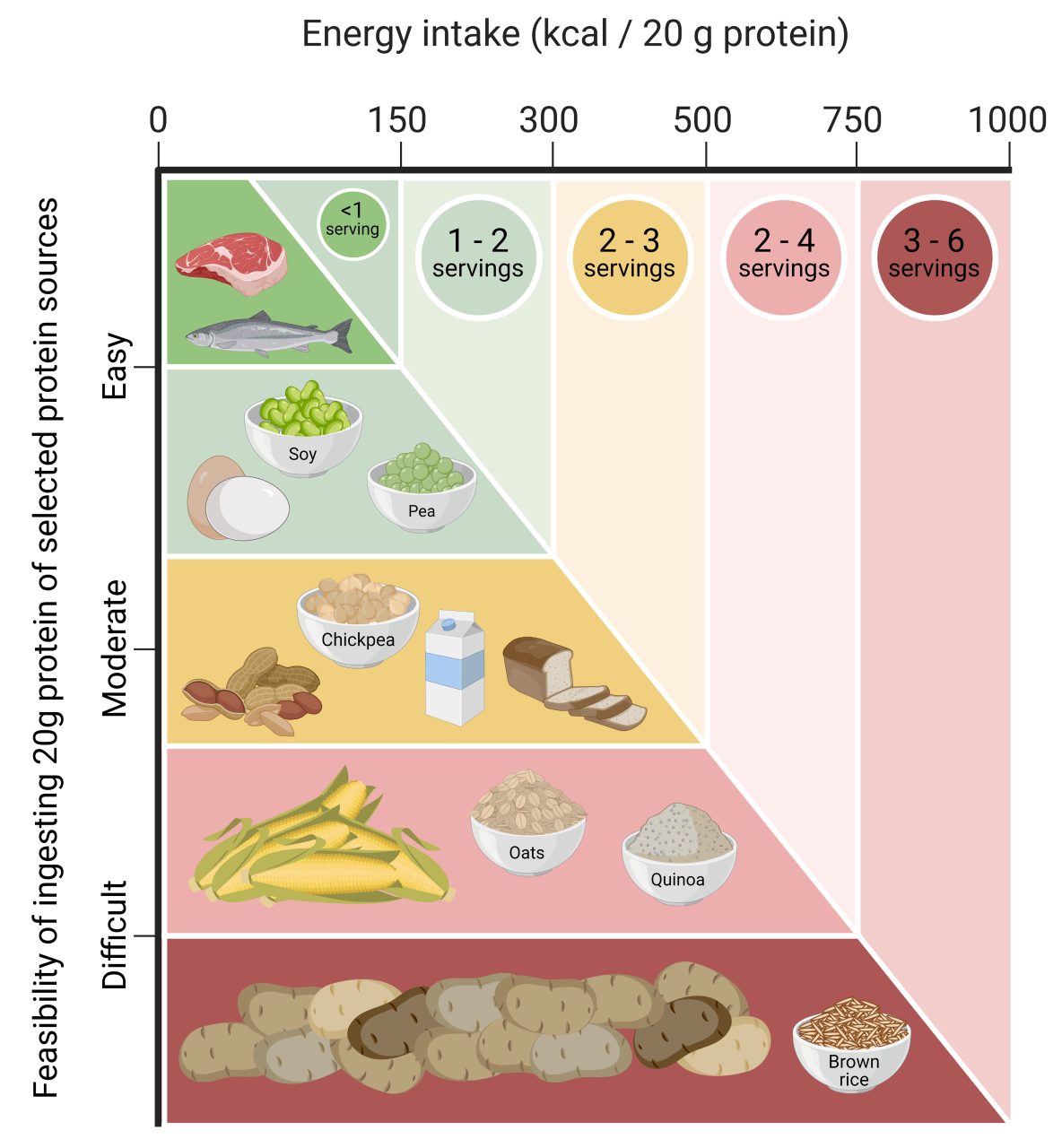
Another strategy to increase the anabolic potential of a low-quality plant-based protein source is to combine different proteins and/or sources to provide a protein blend with a more balanced amino acid profile, without any apparent deficiencies. For example, corn, hemp, brown rice, soy and pea protein are low in lysine and/or methionine content (Gorissen et al., 2018). These deficiencies can be compensated for by consuming up to 4 times more of the same protein. However, when combining corn, hemp, or brown rice protein (low lysine and high methionine content) with an equal amount of soy or pea protein (low methionine and high lysine content), you can create a protein blend with a more balanced amino acid profile that largely compensates for any amino acid deficiencies (Gorissen et al., 2018). Besides exclusive plant-based protein blends, combinations of plant- plus animal-derived proteins may also prove to be of great value as a means to lower animal-derived food consumption without compromising overall protein quality. In support, robust increases in post-prandial muscle protein synthesis rates have been reported following ingestion of whey, casein, and soy protein blends (Reidy et al., 2013). Many more protein blends can be composed to achieve particular aims regarding amino acid composition, price, taste, and sustainability without compromising the capacity to stimulate muscle protein synthesis (Pinckaers et al., 2021).
If a specific amino acid deficiency forms the limiting factor for a plant-based protein (source) to increase muscle protein synthesis, an alternative option would be to fortify the product with the specific (free) amino acid(s). In support, leucine fortification of a bolus of intact protein, amino acid mixture, or mixed meal has been reported to further increase muscle protein synthesis rates (Katsanos et al., 2006; Rieu et al., 2006; Wall et al., 2013). As far as we know, few data are available on the impact of leucine fortification of plant-based proteins as a means to further increase muscle protein synthesis rates. A study in rodents demonstrated lower muscle protein synthesis rates after feeding wheat versus whey protein (Norton et al., 2012). Fortification of the wheat protein with free leucine, to match the leucine content of an equivalent amount of whey protein, increased muscle protein synthesis rates to a level that was no longer different from the response observed after whey protein feeding. In contrast, we did not observe higher post-exercise muscle protein synthesis rates following ingestion of 20 g soy protein fortified with 2.5 g free leucine compared with 20 g soy protein only (Churchward-Venne et al., 2019b). We can only assume that under these conditions leucine content was not a limiting factor for the post-prandial increase in muscle protein synthesis rates. With many plant-based proteins being deficient in lysine and/or methionine, it has been hypothesized that fortification of these plant-based proteins is required to fully unlock their anabolic potential. Though such free amino acid fortification is commonly applied in plant-based products designed to replace meat or dairy products, there are no studies that have assessed the efficacy of free amino acid fortification to (further) augment the muscle protein synthesis rates following the ingestion of such plant-based proteins.
PROTEIN HANDLING FOLLOWING MEAL INGESTION
Work on the anabolic properties of plant-based proteins has been largely confined to a handful of plant-based and animal-derived protein isolates or concentrates. However, dietary protein is generally consumed in the form of a whole food or food product and as part of a more complete, composite meal. When consuming protein as part of a product and/or meal, other nutrients such as carbohydrates, fats, micronutrients, and other (anti-) nutritional compounds may modify post-prandial protein digestion and amino acid absorption kinetics and subsequent muscle protein synthesis rates (Trommelen et al., 2019). In support, we (Gorissen et al., 2014; Koopman et al., 2007) have shown that post-prandial protein digestion and amino acid absorption may be delayed when carbohydrate or fat are co-ingested with protein. However, co-ingestion of carbohydrate with protein does not seem to modulate muscle protein synthesis rates during recovery from exercise (Koopman et al., 2007).
Although such studies provide insight into the impact of co-ingesting other macronutrients on protein digestion and amino acid absorption kinetics and the subsequent post-prandial stimulation of muscle protein synthesis, they do not necessarily reflect the anabolic response to the ingestion of the whole foods from which they are derived. Whereas several studies have assessed post-prandial muscle protein synthesis rates following the ingestion of whole foods such as milk (Burd et al., 2015), meat (Burd et al., 2015; Robinson et al., 2013), and eggs (van Vliet et al., 2017), there is hardly any work done on the anabolic responses to the ingestion of plant-based whole foods or mixed meals. This prevents us from understanding the true anabolic properties of consuming plant-based foods, as the food matrix of plant-based foods may compromise protein digestion and amino acid absorption kinetics and attenuate the postprandial rise in muscle protein synthesis rates. The muscle protein synthetic response to meal ingestion is complex and cannot simply be predicted by the amino acid composition of the protein or the circulating plasma amino acid concentrations.
PLANT-DERIVED PROTEINS IN SPORTS NUTRITION
The transition toward a more plant-based diet has also gained interest among athletes and their coaches. Not surprisingly, this has raised many questions regarding the impact of consuming (lower) quality plant-based proteins on recovery and muscle conditioning following exercise. There are few studies that have compared post-exercise muscle protein synthetic responses following the ingestion of plant- versus animal-derived proteins (Churchward-Venne et al., 2019b; Gorissen et al., 2016; Pinckaers et al., 2021; Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012b). In these studies, the main plant-derived protein that has been applied is soy protein. Some (Tang et al., 2009; Wilkinson et al., 2007; Yang et al., 2012b) but certainly not all (Churchward-Venne et al., 2019b) studies have reported less of an increase in post-exercise muscle protein synthesis rates following ingestion of soy protein when compared with an equivalent amount of milk or whey protein. Exercise makes the muscle more sensitive to the anabolic properties of amino acid or protein administration. Therefore, it could be speculated that the post-prandial rise in circulating plasma leucine concentrations is of lesser importance when protein is consumed during recovery from exercise. The low(er) leucine content of most plant-derived proteins may no longer compromise muscle protein synthesis rates during the acute stages of post-exercise recovery. The capacity of a protein to support the post-exercise increase in muscle protein synthesis will be more likely determined by the amount of amino acids and the rate at which they are provided as precursors for muscle protein synthesis. An ample provision of all amino acids without any deficiencies in specific amino acids may be of primary importance when determining the optimal plant-derived protein (blend) to support post-exercise muscle conditioning.
Long-term intervention studies tend to show greater gains in muscle mass and strength when protein supplementation is applied during prolonged resistance type exercise training (Cermak et al., 2012). Increases in daily muscle protein synthesis rates and/or gains in muscle mass have been reported following resistance type exercise training while supplementing plant-derived proteins, such as soy, pea, rice, and potato protein (Lim et al., 2021). It is unlikely that these gains in muscle mass and strength would have differed much from the gains observed when an equivalent amount of animal-based protein would have been supplemented. A recent meta-analysis concluded that the animal- or plant-based origin of the supplemented protein source does not significantly impact gains in lean mass or muscle strength following resistance type exercise training (Lim et al., 2021). Furthermore, recent work (Hevia-Larraín et al., 2021) reported no differences in muscle mass and strength gains following prolonged resistance exercise training while consuming either an exclusively plant-based diet or an omnivorous diet. This should not be a surprise, as the untrained subjects were consuming a high-protein intake diet (~ 1.6 g protein/kg body mass/day), with substantial amounts of soy or whey protein isolates being supplemented twice daily.
Based upon the described differences in protein absorbability, protein digestion and amino acid kinetics, and amino acid composition between plant- and animal-based protein sources, it seems fair to assume that transitioning toward a more plant-based diet would require more protein to be consumed. However, most athletes already consume ample amounts of protein due to their high energy intake. A nationwide survey of well-trained athletes reported a protein intake of ~1.5 g protein/kg body mass/day (Gillen et al., 2017). Though this represents a daily protein intake well above the Recommended Daily Allowance (RDA), it has been argued that a protein intake of up to 1.6 g/kg body mass/day would maximize gains in muscle mass and strength during prolonged resistance type exercise training. Consequently, it could be speculated that a diet providing low(er) quality protein could compromise the skeletal muscle adaptive response to exercise training. However, the latter represents more an academic concept, as small differences in protein quality will not have much impact on the adaptive response to exercise training when such large amounts of protein are habitually consumed. Furthermore, it should be noted that athletes already derive more than 40% of their habitual daily protein intake from plant-based sources (Gillen et al., 2017). More important is the potential negative impact of transitioning toward a more plant-based diet in a setting where athletes lower their energy intake and, as such, further reduce protein consumption. Athletes trying to reduce body weight by caloric restriction or athletes recovering from an injury would actually require a similar or even higher (absolute) protein intake while consuming less food. In such conditions the protein density of the foods and the quality of the consumed protein become of key importance. Transitioning to a diet with lower anabolic properties could compromise muscle maintenance and attenuate muscle regain. Clearly, we need to evaluate the positive as well as the potential negative aspects of transitioning toward a more plant-based diet. Though there are many health benefits to be expected from a transition toward a more exclusive plant-based diet, we need to evaluate whether this is accompanied by a transition toward greater dietary protein requirements in both health and disease.
OTHER PROTEIN SOURCES
Huge investments are presently being made in the search for a more sustainable production of high-quality protein sources that are not derived from animals. This process has now expanded from plant-derived protein sources to various other potential sources of protein that are fit for human consumption. Such protein sources include yeast, fungi, micro-algae, insects, and even lab-grown meat. Research is ongoing to establish the bioavailability and functionality of many of these novel protein sources. It is likely that many of these alternative protein sources will rapidly find their way into sports nutrition products.
SUMMARY
At present, there is increasing interest in the transitioning toward the consumption of a more plant-based diet. Consuming a plant-based diet will increase the intake of plant-based proteins at the expense of animal-based proteins. So far, research has shown that the ingestion of plant-derived proteins, such as soy and wheat protein, does not stimulate muscle protein synthesis to the same extent as that of an equivalent amount of animal-derived protein. The lower anabolic properties of plant-based proteins compared with animal-derived proteins have been attributed to differences in protein digestion and amino acid absorption kinetics as well as amino acid composition. Most plant-derived proteins have lower essential amino acid content when compared with animal-derived proteins and can be deficient in one or more specific amino acids. Few studies have directly compared muscle protein synthesis rates after the ingestion of plant- versus high(er) quality animal-derived proteins. The lower anabolic properties of plant- versus animal-derived proteins may be compensated for by: consuming more of the plant-derived protein (source), using blends of different plant-based proteins to create a more balanced amino acid profile, and/or by fortifying the plant-based protein (source) with the specific free amino acid(s) that is (are) deficient. More work is required to assess the anabolic properties of different proteins and their protein sources and to identify the factors that may or may not compromise the capacity to stimulate muscle protein synthesis. As healthy, active athletes typically consume a diet that provides well above ~1.5 g protein/day, a lesser protein quality will unlikely compromise muscle conditioning in athletes adopting a (more) plant-based diet. However, when athletes are changing to a plant-based diet under conditions of low energy and/or protein intake, consulting a sports dietician would be recommended to ensure sufficient protein provision.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Boirie, Y., M. Dangin, P. Gachon, M.-P. Vasson, J.-L. Maubois, and B. Beaufrère (1997). Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Nat. Acad. Sci. 94:14930-14935.
Brennan, J.L., M. Keerati-U-Rai, H. Yin, J. Daoust, E. Nonnotte, L. Quinquis, T. St-Denis, and D.R. Bolster (2019). Differential responses of blood essential amino acid levels following ingestion of high-quality plant-based protein blends compared to whey protein—a double-blind randomized, cross-over, clinical trial. Nutrients 11:2987.
Burd, N.A., S.H. Gorissen, S. van Vliet, T. Snijders, and L.J. van Loon (2015). Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am. J. Clin. Nutr. 102:828-836.
Cermak, N.M., P.T. Res, L.C. de Groot, W.H. Saris, and L.J. van Loon (2012). Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am. J. Clin. Nutr. 96:1454-1464.
Churchward-Venne, T.A., P.J.M. Pinckaers, J.S.J. Smeets, W.M. Peeters, A.H. Zorenc, H. Schierbeek, I. Rollo, L.B. Verdijk, and L.J.C. van Loon (2019a). Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. J. Nutr. 149:198-209.
Churchward-Venne, T.A., P.J.M. Pinckaers, J.S.J. Smeets, W.M. Peeters, A.H. Zorenc, H. Schierbeek, I. Rollo, L.B. Verdijk, and L.J.C. van Loon (2019b). Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with whey, soy, or leucine-enriched soy protein after concurrent resistance- and endurance-type exercise. J. Nutr. 149:210-220.
Churchward-Venne, T.A., P.J.M. Pinckaers, J.S.J. Smeets, M.W. Betz, J.M. Senden, J.P.B. Goessens, A.P. Gijsen, I. Rollo, L.B. Verdijk, and L.J.C. van Loon (2020). Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am. J. Clin. Nutr. 112:303-317.
Devi, S., A. Varkey, M.S. Sheshshayee, T. Preston, and A.V. Kurpad (2018). Measurement of protein digestibility in humans by a dual-tracer method. Am. J. Clin Nutr. 107:984-991.
Dickinson, J.M., D.M. Gundermann, D.K. Walker, P.T. Reidy, M.S. Borack, M.J. Drummond, M. Arora, E. Volpi, and R.B. Rasmussen (2014). Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J. Nutr. 144:1694-1702.
FAO/WHO/UNU expert consultation (2007). Protein and amino acid requirements in human nutrition. WHO Technical Report Series 935:1-265.
Gausserès, N., S. Mahé, R. Benamouzig, C. Luengo, F. Ferriere, J. Rautureau, and D. Tomé (1997). [15N]-labeled pea flour protein nitrogen exhibits good ileal digestibility and postprandial retention in humans. J. Nutr. 127:1160-1165.
Gillen, J.B., J. Trommelen, F.C. Wardenaar, N.Y.J. Brinkmans, J.J. Versteegen, K.L. Jonvik, C. Kapp, J. De Vries, J.J.G.C. Van Den Borne, M.J. Gibala, and L.J.C. Van Loon (2017). Dietary protein intake and distribution patterns of well-trained dutch athletes. Int. J. Sport Nutr. Exerc. Metab. 27:105-114.
Gorissen, S.H., N.A. Burd, H.M. Hamer, A.P. Gijsen, B.B. Groen, and L.J. van Loon (2014). Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J. Clin. Endocrinol. Metab. 99:2250-2258.
Gorissen, S.H., D. Remond, and L.J. van Loon (2015). The muscle protein synthetic response to food ingestion. Meat Sci. 109:96-100.
Gorissen, S.H., A.M. Horstman, R. Franssen, J.J. Crombag, H. Langer, J. Bierau, F. Respondek, and L.J. van Loon (2016). Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. J. Nutr. 146:1651-1659.
Gorissen, S.H., J.J. Crombag, J.M. Senden, W.H. Waterval, J. Bierau, L.B. Verdijk, and L.J. van Loon (2018). Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50:1685-1695.
Gorissen, S.H.M., J. Trommelen, I.W.K. Kouw, A.M. Holwerda, B. Pennings, B.B.L. Groen, B.T. Wall, T.A. Churchward-Venne, A.M.H. Horstman, R. Koopman, N.A. Burd, C.J. Fuchs, M.L. Dirks, P.T. Res, J.M.G. Senden, J.M.J.M. Steijns, L.C.P.G.M. De Groote, L.B. Verdijk, and L.J.C. van Loon (2020). Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J. Nutr. 150:2041-2050.
Groen, B.B., A.M. Horstman, H.M. Hamer, M. de Haan, J. van Kranenburg, J. Bierau, M. Poeze, W.K. Wodzig, B.B. Rasmussen, and L.J. van Loon (2015). Post-prandial protein handling: you are what you just ate. PloS One 10:e0141582.
Hevia-Larraín, V., B. Gualano, I. Longobardi, S. Gil, A.L. Fernandes, L.A.R. Costa, R.M.R. Pereira, G.G. Artioli, S.M. Phillips, and H. Roschel (2021). High-protein plant-based diet versus a protein-matched omnivorous diet to support resistance training adaptations: A comparison between habitual vegans and omnivores. Sports Med. 51:1317-1330.
Kashyap, S., N. Shivakumar, A. Varkey, R. Duraisamy, T. Thomas, T. Preston, S. Devi, and A.V. Kurpad (2018). Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am. J. Clin. Nutr. 108:980-987.
Kashyap, S., A. Varkey, N. Shivakumar, S. Devi, B.H.R. Reddy, T. Thomas, T. Preston, S. Sreeman, and A.V. Kurpad (2019). True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am. J. Clin. Nutr. 110:873-882.
Katsanos, C.S., H. Kobayashi, M. Sheffield-Moore, A. Aarsland, and R.R. Wolfe (2006). A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. 291:E381-E387.
Koopman, R., A.J.M. Wagenmakers, R.J.F. Manders, A.H.G. Zorenc, J.M.G. Senden, M.Gorselink, H.A. Keizer, and L.J.C. Van Loon (2005). Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. 288:E645-E653.
Koopman, R., M. Beelen, T. Stellingwerff, B. Pennings, W.H.M. Saris, A.K. Kies, H. Kuipers, and L.J.C. Van Loon (2007). Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am. J. Physiol. 293:E833-E842.
Koopman, R., N. Crombach, A.P. Gijsen, S. Walrand, J. Fauquant, A.K. Kies, S. Lemosquet, W.H. Saris, Y. Boirie, and L.J. van Loon (2009a). Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 90:106-115.
Koopman, R., S. Walrand, M. Beelen, A.P. Gijsen, A.K. Kies, Y. Boirie, W.H. Saris, and L.J. van Loon (2009b). Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 139:1707-1713.
Lim, M.T., B.J. Pan, D.W.K. Toh, C.N. Sutanto, and J.E. Kim (2021). Animal protein versus plant protein in supporting lean mass and muscle strength: a systematic review and meta-analysis of randomized controlled trials. Nutrients 13:661.
Liu, J., M. Klebach, M. Visser, and Z. Hofman (2019). Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: a double-blind, cross-over trial. Nutrients 11:2613.
Moore, D.R., M.J. Robinson, J.L. Fry, J.E. Tang, E.I. Glover, S.B. Wilkinson, T. Prior, M.A. Tarnopolsky, and S.M. Phillips (2009a). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89:161-168.
Moore, D.R., J.E. Tang, N.A. Burd, T. Rerecich, M.A. Tarnopolsky, and S.M. Phillips (2009b). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J. Physiol. 587:897-904.
Norton, L.E., G.J. Wilson, D.K. Layman, C.J. Moulton, and P.J. Garlick (2012). Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. 9:67.
Pennings, B., Y. Boirie, J.M. Senden, A.P. Gijsen, H. Kuipers, and L.J. van Loon (2011). Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 93:997-1005.
Pinckaers, P.J.M., I.W.K. Kouw, F.K. Hendriks, J.M.X. Van Kranenburg, L.C.P.G.M. De Groot, L.B. Verdijk, T. Snijders, and L.J.C. Van Loon (2021). No differences in muscle protein synthesis rates following ingestion of wheat protein, milk protein, and their protein blend in healthy, young males. Br, J. Nutr. 18:1-11.
Reidy, P.T., D.K. Walker, J.M. Dickinson, D.M. Gundermann, M.J. Drummond, K.L. Timmerman, C.S. Fry, M.S. Borack, M.B. Cope, R. Mukherjea, K. Jennings, E. Volpi, and B.B. Rasmussen (2013). Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J. Nutr. 143:410-416.
Rieu, I., M. Balage, C. Sornet, C. Giraudet, E. Pujos, J. Grizard, L. Mosoni, and D. Dardevet (2006). Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 575:305-315.
Robinson, M.J., N.A. Burd, L. Breen, T. Rerecich, Y. Yang, A.J. Hector, S.K. Baker, and S.M. Phillips (2013). Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 38:120-125.
Sarwar Gilani, G., C. Wu Xiao, and K.A. Cockell (2012). Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 108:S315-S332.
Tang, J.E., J.J. Manolakos, G.W. Kujbida, P.J. Lysecki, D.R. Moore, and S.M. Phillips (2007). Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl. Physiol. Nutr. Metab. 32:1132-1138.
Tang, J.E., D.R. Moore, G.W. Kujbida, M.A. Tarnopolsky, and S.M. Phillips (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 107:987-992.
Tipton, K.D., T.A. Elliott, M.G. Cree, S.E. Wolf, A.P. Sanford, and R.R. Wolfe (2004). Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci. Sports Exerc. 36:2073-2081.
Trommelen, J., M.W. Betz, and L.J.C. Van Loon (2019). The muscle protein synthetic response to meal ingestion following resistance-type exercise. Sports Med. 49:185-197.
Trommelen, J., A.M. Holwerda, P.J.M. Pinckaers, and L.J.C. Van Loon (2021a). Comprehensive assessment of post-prandial protein handling by the application of intrinsically labelled protein in vivo in human subjects. Proc. Nutr. Soc. 80:221-229.
Trommelen, J., D. Tomé, and L.J.C. Van Loon (2021b). Gut amino acid absorption in humans: concepts and relevance for postprandial metabolism. Clin. Nutr. 36:43-55.
van Vliet, S., N.A. Burd, and L.J. van Loon (2015). The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J. Nutr. 145:1981-1991.
van Vliet, S., E.I. Shy, S. Abou Sawan, J.W. Beals, D.W. West, S.K. Skinner, A.V. Ulanov, Z. Li, S.A. Paluska, C.M. Parsons, D.R. Moore, and N.A. Burd (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am. J. Clin. Nutr. 106:1401-1412.
Wall, B.T., H.M. Hamer, A. de Lange, A. Kiskini, B.B. Groen, J.M. Senden, A.P. Gijsen, L.B. Verdijk, and L.J. van Loon (2013). Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 32:412-419.
Wilkinson, S.B., M.A. Tarnopolsky, M.J. Macdonald, J.R. Macdonald, D. Armstrong, and S.M. Phillips (2007). Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 85:1031-1040.
Wilkinson, D.J., T. Hossain, D.S. Hill, B.E. Phillips, H. Crossland, J. Williams, P. Loughna, T.A. Churchward-Venne, L. Breen, S.M. Phillips, T. Etheridge, J.A. Rathmacher, K. Smith, N.J. Szewczyk, and P.J. Atherton (2013). Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 591:2911-2923.
Wilkinson, D.J., S.S.I. Bukhari, B.E. Phillips, M.C. Limb, J. Cegielski, M.S. Brook, D. Rankin, W.K. Mitchell, H. Kobayashi, J.P. Williams, J. Lund, P.L. Greenhaff, K. Smith, and P.J. Atherton (2018). Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 37:2011-2021.
Witard, O.C., S.R. Jackman, L. Breen, K. Smith, A. Selby, and K.D. Tipton (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 99:86-95.
Yang, Y., L. Breen, N.A. Burd, A.J. Hector, T.A. Churchward-Venne, A.R. Josse, M.A. Tarnopolsky, and S.M. Phillips (2012a). Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 108:1780-1788.
Yang, Y., T.A. Churchward-Venne, N.A. Burd, L. Breen, M.A. Tarnopolsky, and S.M. Phillips (2012b). Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 9:57.








