FROM CELIAC DISEASE, GLUTEN-SENSITIVITY VS GLUTEN SENSATIONALISM, TO FODMAP REDUCTION AS A TOOL TO MANAGE GASTROINTESTINAL SYMPTOMS IN ATHLETES

KEY POINTS
- Exercise-induced gastrointestinal symptoms are commonly reported by athletes.
- A gluten-free diet (GFD) for athletes not clinically requiring gluten avoidance has not been shown to have a beneficial or negative effect on gastrointestinal health, overall well-being or athletic performance.
- Athletes may effectively, yet unknowingly, eliminate foods high in fermentable oligo-, di- and monosaccharides and polyols (FODMAPs) with the aim to reduce gastrointestinal symptoms.
- Strategically applied, a low FODMAP diet may be an effective strategy to reduce the occurrence or intensity of exercise-induced gastrointestinal symptoms.
EXERCISE-INDUCED GASTROINTESTINAL SYNDROME AND DIET
Among individuals participating in endurance sport, gastrointestinal symptoms (GIS) are frequently reported from mild to severe. Moderate and severe GIS have been reported to occur at varying rates; 27% in marathon, 32% in Ironman and up to 60-96% in longer endurance events (e.g., ultra-marathon running), potentially compromising performance (Costa et al., 2016; Pfeiffer et al., 2012; Pugh et al., 2018; Stuempfle & Hoffman, 2015). Endurance athletes are the most researched and relevant; however, the details of this SSE are applicable to many other athletes experiencing GI symptoms for a variety of reasons. GIS associated with exercise are multifactorial in nature, transient and difficult to replicate. The primary triggers for the array of upper and lower GIS are suggested to stem from a condition recently termed exercise-induced gastrointestinal syndrome (EIGS). Although exercise stress is likely to control the degree of disruption to the GI tract, numerous dietary factors may influence the incidence and intensity of GIS before, during and after exercise. Nutritional strategies including appropriate meal timing, low fiber/residue, low fat and moderate protein intakes are the generally established strategies implemented to reduce GIS.
One of the most recent and popular diets has been a gluten-free diet (GFD), which has exploded in popularity among non-celiac athletes as a resolution for exercise-associated GIS, yet supportive data is lacking, with mostly anecdotal evidence supporting its efficacy (Lis et al., 2015b; Newberry et al., 2017). Several dietary changes, subsequent to the avoidance of gluten-containing foods, may occur and influence the perceived beneficial effects of this diet, which extend beyond improvements in gut health (Lis et al., 2015b). Most notable of these dietary changes is a subsequent reduction in fermentable oligo-, di- and monosaccharides and polyols (FODMAPs), mainly fructans (Gibson et al., 2015). Reduced intakes of these poorly digested short-chain carbohydrates before, during and potentially after endurance exercise may be more effective than gluten-avoidance to address GIS commonly linked to exercise in susceptible individuals (Gibson et al., 2015; Skodje et al., 2018). Given the widespread uptake of GFDs for athletes not clinically requiring this diet and the risks associated with unnecessary dietary restriction, education concerning the appropriateness of gluten-avoidance and other possible nutritional considerations that are likely to modulate GIS is pertinent. This Sports Science Exchange article will examine the foundations of GIS in athletes and the current state of knowledge about GFDs for athletes not requiring this diet, but crediting gluten as the cause of GIS. Further, the link between avoidance of wheat-based foods, GIS improvement and FODMAPs will be discussed in an athletic context.
MAIN TRIGGERS OF EXERCISE-INDUCED GASTROINTESTINAL SYNDROME
A general appreciation of the two main physiological alterations induced with exercise stress is central for considering how dietary triggers may influence GIS severity and the intricacies of navigating these changes. First, strenuous exercise (e.g., cycling at 70% Wmax for 45 min) reduces blood flow to the organs of the GI tract; termed splanchnic hypoperfusion. As blood flow is shunted away from the organs of the abdominal cavity to the contracting muscles and peripheral circulation, an insufficient blood supply to the GI tract results in ischemia (van Wijck et al., 2012). Subsequent injury to epithelial cells may reduce their functional capabilities, increase gut barrier permeability, surge inflammatory responses and promote epithelial dysregulation (Dokladny et al., 2016; Zuhl et al., 2014). Secondly, increased activity of the neuroendocrine-gastrointestinal pathway and elevated sympathetic nervous system activation may reduce gastric emptying and delay orocecal transit (Costa et al., 2017a). Evidence further suggests, that as a result of exercise stress and GI dysfunction, some nutrients are malabsorbed, thus adding additional complexity to fueling strategies (Costa et al., 2017b; Lang et al., 2006; van Wijck et al., 2013).
GLUTEN AND GASTROINTESTINAL SYMPTOMS
An array of evidence-based and anecdotal beliefs exists about diets, nutritional supplements, food and pre-exercise behaviors aimed at preventing or reducing exercise-associated GIS. Among these, gluten-avoidance has gained an exceeding popularity within athlete groups (see box below for GFD definition). Most recently, an international study of just under one thousand athletes (n=910) found that 41% reported adhering to a GFD to varying degrees (Lis et al., 2015b). However, evidence-based data to support this are lacking and only anecdotal substantiation supports its efficacy. It is beyond the scope of this article to discuss the specifics of clinical conditions requiring gluten avoidance. Nonetheless, the number of athletes reported to follow a GFD appears to be ~4-fold higher than the estimated general population clinically requiring a GFD (Caio et al., 2017). Gluten-avoidance is essential in managing signs, symptoms and health of individuals with clinical conditions such as celiac disease, wheat allergy and genuine non-celiac gluten sensitivity. Conversely, in clinically healthy athletes (or athletes self-diagnosing gluten-related conditions) there is the belief that a GFD also offers the same health benefits. While this has not been shown, it is interesting to consider the unique element of athletes training at high intensities who experience repeated stress placed on the gut. Theoretically, a persistent state of various levels of gut injury may increase sensitivity to known dietary triggers or associate with the development of chronic disease (e.g., functional GI disorders) acutely or after repeated occurrences (Costa et al., 2017a). Due to the current lack of a diagnostic biomarker for non-celiac gluten sensitivity and an arduous diagnosis process, many athletes self-diagnose this condition and subsequently adopt a GFD without accurately establishing underlying mechanisms.
|
Gluten-free diet: A strict GFD eliminates all sources of gluten, a storage protein composite, with the alcohol-soluble gliadins defined as prolamins and the alcohol-insoluble glutenins as glutelins. There are several clinical conditions requiring a gluten-free diet. These include celiac disease, wheat allergy, gluten ataxia and non-celiac gluten sensitivity (Newberry et al., 2017; Vici et al., 2016). |
Beyond GI symptoms, a GFD, even without a clinical necessity, is believed to improve GI health and symptoms, reduce inflammation, be an overall healthier diet and offer an ergogenic edge (Lis et al., 2015b). To date, the only comprehensive study has been conducted in non-celiac athletes. In this randomized crossover double-blind study, non-celiac cyclists followed a short-term gluten-containing or gluten-free diet with a 10 day wash-out period between interventions. Notwithstanding the tightly controlled and replicated diet and exercise regimens, no difference in measures of GI injury, GIS, systemic inflammatory responses, perceptual well-being or individual time trial performance was observed between the diets (Lis et al., 2015a). While in many athlete groups the benefits of gluten-avoidance continue to be hyped, a GFD independent of clinical conditions has not been shown to have beneficial or negative effects (Lis et al., 2015a). For future management of this diet in clinically healthy athletes it is also practical to consider other dietary changes likely occurring alongside gluten-avoidance, which may play a role in influencing the perceived or real benefits of this diet (Figure 1).
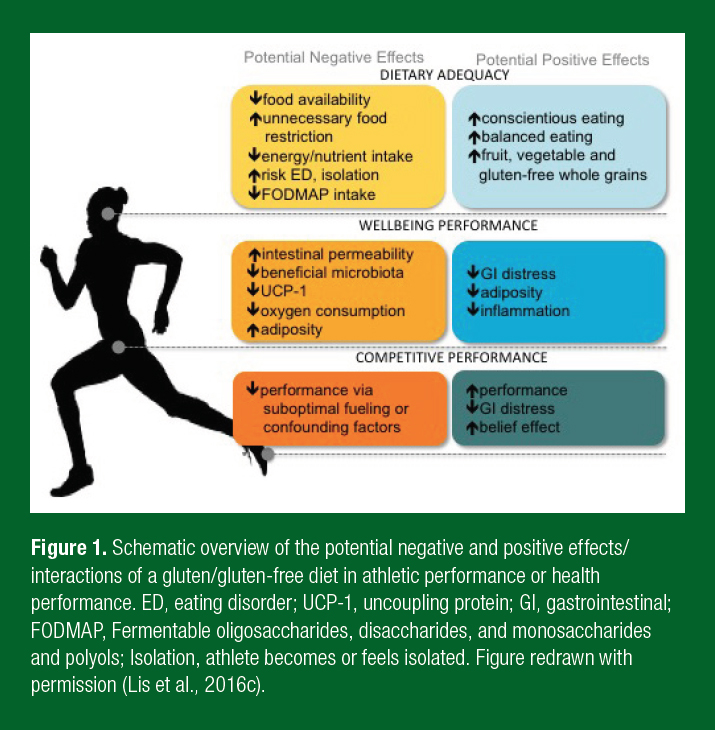
DIETARY CONSIDERATIONS OF GFD FOR ATHLETES
Risks are associated with self-diagnosis of gluten-related clinical conditions and subsequent adoption of a GFD, as the potential underlying medical or physiological conditions could be overlooked. Several nutritional challenges have also been associated with the avoidance of gluten-containing foods (Figure 1). Of particular importance to athletes is the potential risk of unnecessary food restriction compromising energy and nutrient intake and underlying restrictive eating behavior or eating disorders (Cialdella-Kam et al., 2016; Hill et al., 2017). While the gluten-free food market has dramatically enhanced the quality and quantity of gluten-free foods in many parts of the world, concern remains pertaining to reduced prebiotic intake and the concomitant impacts on gut microbiota (Gaesser & Angadi, 2012; Vici et al., 2016). Conversely, several positive elements have been anecdotally ascribed to a GFD. Athletes indicate that a GFD improved overall dietary balance and intake of fruits, vegetables and gluten-free whole grains. These are all underpinning principles of healthy sports nutrition foundations (Lis et al., 2015b). These positive dietary habits may be attributed to simply ascribing to nutrition guidelines and may not be sustainable long-term. Therefore, the appropriateness of a GFD should be individually considered and guided by a qualified and evidence-influenced nutritional professional (e.g., registered dietitian specializing in sports nutrition (Lis et al., 2016c)). Unfortunately, non-scholarly advice is likely more instrumental in influencing athletes’ GFD decisions and often lacks the individualization required to optimize dietary intake supporting peak athletic performance (Lis et al., 2015b).
THE GFD TO FODMAP LINK
An evidence-based systematic approach to uncovering the appropriateness of GFD adherence may help to identify exact triggers for GIS and related symptoms. Interestingly, the intake of FODMAPs, specifically fructans, is reduced or eliminated alongside avoidance of gluten-free grains. It is quite possible that a reduction in FODMAP load is the modulating factor for symptom improvement on a GFD (avoidance of wheat-based grains), and not gluten itself (Gibson et al., 2015; Skodje et al., 2018). This concept is strongly supported by several clinical studies and scientific editorials suggesting that a reduction in FODMAP intake is accountable for improvements in GIS, and that gluten itself is not the modulating factor (Gibson et al., 2015; Hill et al., 2017; Skodje et al., 2018). Skodje et al. (2018) recently demonstrated that fructans triggered GIS akin with irritable bowel syndrome (IBS) in twice as many individuals with self-reported non-celiac gluten sensitivity than gluten. A low-FODMAPs diet is used as a frontline strategy for the treatment of symptomatic IBS with 70% of individuals with IBS reporting successful symptom reduction (Staudacher et al., 2017). Considering that endurance athletes experience symptoms analogous to the set of upper and lower GIS representative of IBS (e.g., intragastric pressure, excessive flatulence, lower abdominal bloating and pain, urge to defecate, alterations in bowel movement and abnormal loose/watery stool), strategic FODMAP reduction is emerging as a promising dietary tool to address exercise-associated GIS in clinically healthy endurance athletes (Costa et al., 2017a; Lis et al., 2016b; 2017; Masuy et al., 2018).
|
FODMAP: Fermentable oligo-, di- and monosaccharides and polyols (FODMAPs) are short-chain carbohydrates that are variably absorbed in the small intestine. They are widespread in the diet and comprise a monosaccharide (fructose), a disaccharide (lactose), oligosaccharides (fructans and galactans) and polyols. FODMAPs are variably/poorly digested, increasing delivery of readily fermentable substrate and water to the distal small intestine and proximal colon, which are likely to induce luminal distension and induction of functional gut symptoms (Gibson & Shepherd, 2010). |
In a healthy general population FODMAPs may not be well digested, but the implications are non-existent or negligible (Ong et al., 2010; Staudacher et al., 2017). As described above, in athletes undertaking strenuous exercise and experiencing an associated impairment of GI function undigested food molecules may increase the osmotic load in the small intestine and contribute to increased osmotic water translocation, volume and physiological consequences such as loose stool or diarrhea (Gibson & Shepherd, 2010). FODMAP intake may also augment GIS initiated by the osmotic effects of high carbohydrate intake necessary to support energy demands (Jeukendrup & McLaughlin, 2010). Upon transit to the lower intestine, malabsorbed and highly fermentable food carbohydrates reach the colon and are subject to bacterial fermentation (breakdown), potentially increasing luminal pressure by rising colonic gas production and volume (e.g., H2, CH4, CO2and H2S) and osmotic water translocation. Resulting symptoms include bloating, lower abdominal pressure and pain, urge to defecate, etc. Lower GIS are more commonly reported among athletes, especially runners. Nonetheless, upper GIS such as sensations of fullness or bloating are also often testified. Until recently, the punitive effects of FODMAPs on the upper GI tract have been minimally investigated. A recent clinical study used a gastric infusion technique to feed fructose and glucose into the stomach of IBS patients as well as healthy controls and measured intragastric pressure, GIS and psychosomatic well-being (Masuy et al., 2018). Postprandial gastric pressure was higher with fructose compared to glucose in both groups alongside higher symptoms scores in the IBS group for bloating, cramps, abdominal pain and flatulence (Masuy et al., 2018). While clinical findings may not be directly transferable to healthy athletes, the symptom resemblances and common alterations in GI function allow for substantial crossover of research findings to related management tools (Lis et al., 2017; Masuy et al., 2018).
LOW FODMAP DIET RESEARCH IN ATHLETES
A handful of studies have examined the impact of FODMAP intake on GIS in clinically healthy athletes. As anecdotally discussed within sport science groups, athletes often implement strategies ahead of the research curve. Supporting this concept, preliminary questionnaire data quantified that 55% of 910 athletes eliminated at least one high FODMAP food with the aim to reduce GIS and 83% of this group of 501 athletes reported successful symptom reduction upon removal of the offending food or foods (Lis et al., 2016a). Supportive case study reports and one published intervention study further encourage the use of a low FODMAP diet compared to a habitual diet (normally high in FODMAPs) to positively affect GIS (Gaskell & Costa, 2018; Lis et al., 2016b). A seminal study examining the role of FODMAP load in GIS in healthy runners demonstrated that 82% of runners (9/11) with persistent self-reported exercise-associated GIS experienced significantly lower daily GIS (outside of exercise) during the low FODMAP compared to a high FODMAP intervention period (Lis et al., 2017). Interestingly, GIS during the prescribed strenuous running bouts were not significantly different and this may be due to the prescribed exercise requiring more length or intensity to distinguish differences in symptoms between the diets. In addition, GIS sometimes become apparent after a strenuous training session/race potentially influencing refueling for subsequent events. GIS symptoms outside of an exercise bout are of concern for events such as track and field where competition occurs multiple times per day or is carried over multiple days. Although GIS are difficult to replicate and transient in nature, emerging evidence is undoubtedly pointing toward a beneficial role of FODMAP modulation for some athletes with EIGS.
LOW FODMAP DIET FOR CLINICALLY HEALTHY ATHLETES
Current methodologies implement a 1-3 day, low FODMAP diet, prior to strenuous exercise or competition/event (Costa et al., 2017b; Stuempfle & Hoffman, 2015). The rationale for this acute period is that FODMAPs would be expected to clear the GI tract in this timeframe. For studies investigating nutritional and exercise interventions on GIS, a low FODMAP lead-in diet is thought to create a uniform baseline diet where minimal GIS would be triggered or augmented by the background FODMAP load. Quantification of a low FODMAP diet for a clinically healthy athlete (e.g., without a functional GI disorder such as IBS) has not been established. Recommendations of < 0.5 g FODMAPs/meal are based on clinical studies conducted in an IBS population (Varney et al., 2017). It is reasonable that in athletes, especially endurance athletes, FODMAP intakes are much higher than values used to classify a high and low FODMAP diet in clinical settings (Ong et al., 2010), thus guidelines would be diverse. Lis et al. (2017) demonstrated that habitual FODMAP intake in runners with self-reported EIGS was 2-fold higher than clinically classified as a high FODMAP diet (43 g/day). Habitual FODMAP intake in endurance athletes may be significantly higher than the average population due to increased overall food intake; high intakes of wheat-based grains/energy bars; high intake of fruits and vegetables; protein bars; cow’s milk-based dairy as a source of quality protein; and sport food products to meet energy and nutrient demands and due to the logistical intricacies of fueling for sport. A low FODMAP diet can be initially challenging to navigate and quite restrictive. It is therefore recommended that athletes considering FODMAP reduction to reduce GIS should consult with a properly trained nutrition professional (e.g., Registered Dietitian, specializing in sport with the low FODMAP diet training certification). Figure 2 suggests a brief implementation pathway for a low FODMAP diet approach in athletes with EIGS. This suggested pathway needs to be individualized and will likely evolve rapidly alongside emerging research.
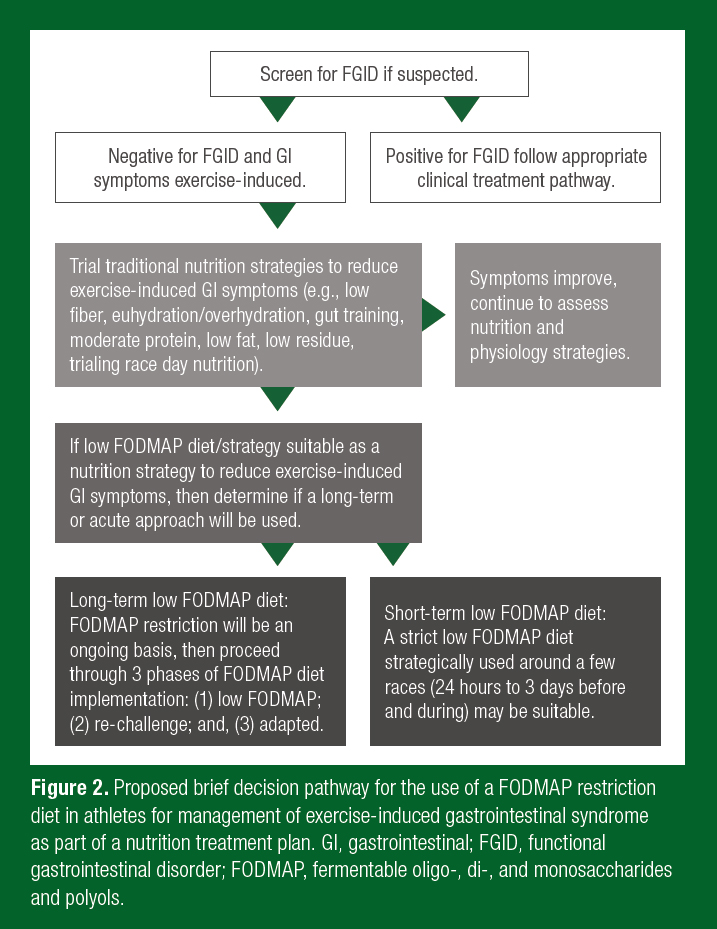
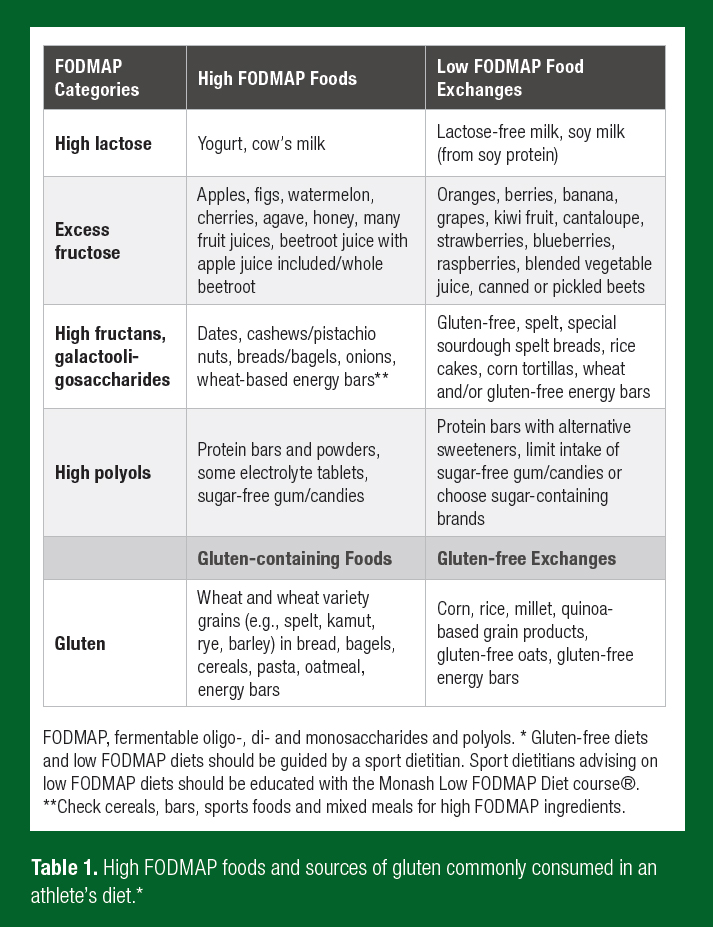
Athletes are very diverse from a clinical population, which has been the primary focus of low FODMAP diet research. However, athletes are susceptible to many of the same potential drawbacks of a long-term and strict low FODMAP diet, as discussed in clinical research, and these should be evaluated before and throughout implementation of this diet (Hill & Gibson, 2017; Lis et al., 2017). Depending on the comprehensiveness of high FODMAP food restriction, several complications associated with a long-term low FODMAP diet have been identified. Strict FODMAP reduction may be associated with alterations in gut microbiota, reduced short chain fatty acid production, as well as effects on the physical and psychological aspects of well-being, similar to those mentioned above with regards to a GFD (Halmos et al., 2015; Lis et al., 2017; Staudacher et al., 2017). A beneficial effect of exercise on gut microbiota has been demonstrated but it is unknown if this is protective against the diminished concentrations of bifidobacteria that are found after 3-4 weeks of reduced FODMAP intake (Lambert et al., 2015; Staudacher et al., 2017). Furthermore, co-administration of a multistrain probiotic, a common supplement used by athletes, has also shown efficacy to mitigate the detrimental effects of lowered prebiotic fiber intake (Staudacher et al., 2017). Reduced prebiotic intake may additionally influence short-chain fatty acid production which is highly reliant upon fermentation of undigested carbohydrates in the large intestine (Wong et al., 2006). The importance of athlete gut health is becoming more apparent with increasing recognition of the multitude of dietary, psychological and environmental factors that influence the composition and metabolic activity of the gut microbiota and interconnected health parameters. Ingestion of short-chain carbohydrates is only one of these components. It is prudent to consider this aspect of gut health when implementing frequent or long-term adherence to FODMAP restriction. In addition, excessive dietary constraint and adherence to restrictive diets are attributed to restrictive eating practices (e.g., orthorexia nervosa) and an increased risk of eating disorders (Cialdella-Kam et al., 2016; Hill et al., 2017), which can be injurious to athletes’ health and performance (Mountjoy et al., 2018). This concept is fundamental when adhering to any diet with the aim to improve health, as it has the potential to do more harm than good by cultivating dietary, nutritional and psychosocial complications. The primary goals of implementing a GFD or low FODMAP diet to manage GIS should be to establish underlying mechanisms and to minimize unnecessary food restriction and the associated psychosocial/nutrition risks. Gluten-containing and high FODMAP foods common in an athlete’s diet and suitable low FODMAP food exchanges are highlighted in Table 1.
PRACTICAL CONSIDERATIONS
- Aim to properly diagnose a gluten-related condition and correct dietary trigger(s) (e.g., FODMAPs/fructans) before unnecessarily choosing a low FODMAP or GFD diet.
- Some athletes with EIGS may benefit from a modified low FODMAP diet. Dietitian-led support from trained practitioners specializing in sport nutrition and the low FODMAP diet may facilitate efficacy, proper use of the diet, and avoid unnecessary restriction or complications associated with food avoidance.
- Athletes benefiting from FODMAP reduction likely require modified dietary planning involving specific high FODMAP foods and not the strictest form of the diet. For example, lactose or fructose may be the most common triggers, and reduction of only high lactose and excess fructose may be needed for symptom improvement. Alternately, employing an acute and strict low FODMAP diet for 1-3 days before and during intensive endurance exercise may be required for sufficient symptom reduction.
- For optimal nutrient delivery (carbohydrate repletion) in the recovery phase, avoidance of high FODMAP foods may also be merited.
- Attention to the potential of unnecessary food restriction that contributes to the development of eating disorders must be carefully navigated when using a GFD or low FODMAP diet.
REFERENCES
Caio, G., G. Riegler, M. Patturelli, A. Facchiano, D.E. Magistris, and A. Sapone (2017). Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. Dietol. 63:16-21.
Cialdella-Kam, L., D. Kulpins, and M.M. Manore (2016). Vegetarian, gluten-free, and energy restricted diets in female athletes. Sports 4:50.
Costa, R.J., R. Snipe, V. Camoes-Costa, V. Scheer, and A. Murray (2016). The impact of gastrointestinal symptoms and dermatological injuries on nutritional intake and hydration status during ultramarathon events. Sports Med. Open 2:16.
Costa, R.J.S., R.M.J. Snipe, C.M. Kitic, and P.R. Gibson (2017a). Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 46:246-265.
Costa, R.J.S., A. Miall, A. Khoo, C. Rauch, R. Snipe, V. Camoes-Costa, and P. Gibson (2017b). Gut-training: The impact of two weeks repetitive gut-challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl. Physiol. Nutr. Metab. 42:547-557.
Dokladny, K., M.N. Zuhl, and P.L. Moseley (2016). Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 120:692-701.
Gaesser, G.A., and S.S. Angadi (2012). Gluten-free diet: Imprudent dietary advice for the general population? J. Acad. Nutr. Diet. 112:1330-1333.
Gaskell, S.K., and R.J.S. Costa (2018). Applying a low-fodmap dietary intervention to a female ultra-endurance runner with irritable bowel syndrome during a multi-stage ultra-marathon. Int. J. Sport Nutr. Exerc. Metab. 14: Epub ahead of print.
Gibson, P.R., and S.J. Shepherd (2010). Evidence-based dietary management of functional gastrointestinal symptoms: The fodmap approach. J. Gastroenterol. Hepatol. 25:252-258.
Gibson, P.R., J.G. Muir, and E.D. Newnham (2015). Other dietary confounders: Fodmaps et al. Dig. Dis. 33:269-276.
Halmos, E.P. C.T. Christophersen, A.R. Bird, S.J. Shepherd, P.R. Gibson, and J.G Muir (2015). Diets that differ in their fodmap content alter the colonic luminal microenvironment. Gut 64:93-100.
Hill, P., J.G. Muir, and P.R. Gibson (2017). Controversies and recent developments of the low-fodmap diet. Gastroenterol. Hepatol. 13:36-45.
Jeukendrup, A.E., and J. McLaughlin (2010). Carbohydrate ingestion during exercise: Effects on performance, training adaptations and trainability of the gut. Nestle Nutr. Inst. Workshop Ser. 69:1-12.
Lambert, J.E., J.P. Myslicki, M.R. Bomhof, D.D. Belke, J. Shearer, and R.A. Reimer (2015). Exercise training modifies gut microbiota in normal and diabetic mice. Appl. Physiol. Nutr. Metab. 40:749-752.
Lang, J.A., C.V. Gisolfi, and G.P. Lambert (2006). Effect of exercise intensity on active and passive glucose absorption. Int. J. Sport Nutr. Exerc. Metab. 16:485-493.
Lis, D., T. Stellingwerff, C.M. Kitic, K.D. Ahuja, and J. Fell, (2015a) No effects of a short-term gluten-free diet on performance in non-celiac athletes. Med. Sci. Sports Exerc. 47:2563-2570.
Lis, D., T. Stellingwerff, C.M. Shing, K.D.K. Ahuja, and J. Fell (2015b). Exploring the popularity, experiences, and beliefs surrounding gluten-free diets in nonceliac athletes. Int. J. Sport Nutr. Exerc. Metab. 25:37-45.
Lis, D., K.D. Ahuja, T. Stellingwerff, C.M. Kitic, and J. Fell (2016a). Food avoidance in
athletes: Fodmap foods on the list. Appl. Physiol. Nutr. Metab. 41:1002-1004.
Lis, D.M., K.D. Ahuja, T. Stellingwerff, C.M. Kitic, and J. Fell (2016b). Case study: Utilizing a
low fodmap diet to combat exercise-induced gastrointestinal symptoms: Med. Sci. Sports
Exerc. 48:965 (abstract).
Lis, D., J.W. Fell, K.D.K. Ahuja, D.K. Kiran., C. Kitic, and T. Stellingwerff (2016c).
Commercial hype versus reality: our current scientific understanding of gluten and
athletic performance. Curr. Sports Med. Rep. 15:262-268.
Lis, D.M., T. Stellingwerff, C.M. Kitic, J.W. Fell, and K.D.K. Ahuja (2017). Low fodmap: A
preliminary strategy to reduce gastrointestinal distress in athletes. Med. Sci. Sports Exerc.
50:116-123.
Masuy, I., L.Van Oudenhove, J. Tack, and J.R. Biesiekierski (2018). Effect of intragastric
fodmap infusion on upper gastrointestinal motility, gastrointestinal, and psychological
symptoms in irritable bowel syndrome vs healthy controls. Neurogastroenterol. Motil.
30: Epub ahead of print.
Mountjoy, M., J.K. Sundgot-Borgen, L.M. Burke, K.E. Ackerman, C. Blauwet, N. Constantini, C. Lebrun, B. Lundy, A.K. Melin, N.L. Meyer, R.T. Sherman, A.S. Tenforde, M. Klungland, M. Torstveit, and R. Budgett (2018).IOC consensus statement on relative energy deficiency in sport (red-s): 2018 update. Br. J. Sports Med. 52:687-697.
Newberry, C., L. McKnight, M. Sarav, and O. Pickett-Blakely (2017). Going gluten free: The history and nutritional implications of today's most popular diet. Curr. Gastroenterol. Rep. 19:54.
Ong, D.K., S.B. Mitchell, J.S. Barrett, S.J. Shepherd, P.M. Irving, J.R. Biesiekierski, S. Smith, P.R. Gibson, and J.G. Muir (2010). Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 25:1366-1373.
Pfeiffer, B., T. Stellingwerff, A.B. Hodgson, R. Randell, K. Pottgen, P. Res, and A.E. Jeukendrup (2012). Nutritional intake and gastrointestinal problems during competitive endurance events. Med. Sci. Sports Exerc. 44:344-351.
Pugh, J.N., B. Kirk, R. Fearn, J.P. Morton, and G.L. Close (2018). Prevalence, severity and potential nutritional causes of gastrointestinal symptoms during a marathon in recreational runners. Nutrients 10:7.
Skodje, G.I., V.K. Sarna, I.H. Minelle, K.L. Rolfsen, J.G. Muir, P.R. Gibson, M.B. Veierod, C. Henriksen, and K.E.A. Lundin (2018). Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 155:228.
Staudacher, H.M., M.C.E. Lomer, F.M. Farquharson, P. Louis, F. Fava, E. Franciosi, M. Scholz, K.M. Tuohy, J.O. Lindsay, P.M. Irving, and K. Whelan (2017). A diet low in fodmaps reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: A randomized controlled trial. Gastroenterology 153:936-947.
Stuempfle, K.J., and M.D. Hoffman (2015). Gastrointestinal distress is common during a 161-km ultramarathon. J. Sports Sci. 33:1814-1821.
van Wijck, K., K. Lenaerts, J. Grootjans, K.A, Wijnands, M. Poeze, L.J. van Loon, C.H. Dejong, and W.A. Buurman (2012). Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. 303:G155-G168.
van Wijck, K., B. Pennings, A.A. van Bijnen, J.M. Senden, W.A. Buurman, C.H. Dejong, L.J. van Loon, and K. Lenaerts (2013). Dietary protein digestion and absorption are impaired during acute postexercise recovery in young men. Am. J. Physiol. 304:R356-R361.
Varney, J., J. Barrett, K. Scarlata, P. Catsos, P.R. Gibson, and J.G. Muir (2017). Fodmaps: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 32(Suppl 1):53-61.
Vici, G., L. Belli, M. Biondi, and V. Polzonetti (2016). Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 35:1236-1241.
Wong, J.M., R. de Souza, C.W. Kendall, A. Emam, and D.J. Jenkins (2006). Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40:235-243.
Zuhl, M., S. Schneider, K. Lanphere, C. Conn, K. Dokladny, and P. Moseley (2014). Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 48:980-986.








