KEY POINTS
- Gastrointestinal (GI) problems are very common, especially in endurance athletes, and often impair performance or subsequent recovery.
- Blood flow to the GI tract is impaired during exercise and this is believed to contribute to the development of GI symptoms.
- There are three main causes of GI symptoms: physiological (reduced blood flow to the gut), mechanical (bouncing effect of running, for example) or nutritional.
- The gut is sensitive to water and nutrient intake during exercise and to hypovolemia, hyperthermia, hypoglycemia, hypoxia and ischemia.
- The information that is available suggests that gut permeability can be compromised in athletes; however, this has not yet been linked conclusively to GI symptoms.
- GI symptoms among athletes vary enormously, and some athletes are more prone than others.
- Nutritional training and appropriate nutrition choices can reduce the risk of GI discomfort during exercise by assuring rapid gastric emptying and absorption of water and nutrients, and by maintaining adequate perfusion of the splanchnic vasculature.
- Avoiding protein, fat, fiber and milk products can reduce the risk of developing GI symptoms during exercise.
INTRODUCTION
Gastrointestinal (GI) complaints are very common among endurance athletes. Anecdotally, GI problems are perhaps the most common cause of underperformance in endurance events. Depending on the methodology used and the events studied, an estimated 30% to 90% of distance runners experience intestinal problems related to exercise. These complaints may be of varying severity, but symptoms may include nausea, vomiting, abdominal angina and bloody diarrhea. In many cases, these problems can have negative effects on performance and also have an impact on subsequent recovery. Bill Rodgers, a marathon legend, with four victories in both the Boston and New York City Marathons in the late 1970s, said, “More marathons are won or lost in the portable toilets than at the dinner table.” This illustrates the magnitude of the problem for endurance athletes and in particular long distance runners. This review will discuss the prevalence of GI complaints in athletes, discuss the etiology of the problems and start to develop guidelines to prevent the issues.
Prevalence of GI problems in athletes
One review stated that in exhausting endurance events, 30% to 50% of participants may suffer from one or more GI symptom (Brouns & Beckers, 1993). A study in long distance triathletes who competed in extreme conditions demonstrated a prevalence of up to 93% for any one GI symptom (Jeukendrup et al., 2000). More alarming was that 43% of triathletes reported serious GI problems and 7% abandoned the race because of GI problems (Jeukendrup et al., 2000). Among elite endurance athletes the prevalence of exercise-induced GI symptoms was reported to be 70% (Peters et al., 1999) and in an Internet-based observational study in 1,281 athletes, 45% reported at least one GI symptom (Ter Steege et al., 2008). Pfeiffer et al. (2011) reported severe GI distress ranging from 4% in marathon running and cycling up to 32% in Ironman races. It was demonstrated that there was a strong correlation between GI symptoms and having a history of GI symptoms (Pfeiffer et al., 2009; Pfeiffer et al., 2011), indicating that some people are more prone to develop GI symptoms and suggesting that there is a large genetic component to these problems. Clearly, there is large variation in the reported prevalence in the literature and this seems to be attributable at least in part to the method of investigation (the way GI symptoms are defined and recorded). In addition, however, the reported prevalence of these symptoms varies in different studies depending on the study population, sex, age and training status of the athletes, as well as mode and intensity of the exercise studied, and the environmental conditions.
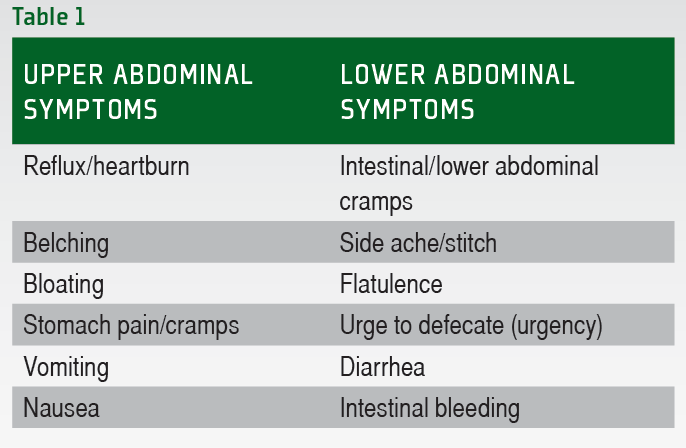
When analyzing the reported symptoms it becomes immediately obvious that these symptoms are highly individual and there are no clear patterns with regards to the type of activity and the types of symptoms observed. There is a fairly large, yet well-defined number of different GI symptoms that can occur during exercise. These symptoms are summarized in Table 1. Generally the symptoms can be classified as either upper or lower GI tract symptoms. Typically lower GI tract problems are more severe in nature, but all symptoms have the potential to impair performance.
Symptoms are often mild and may not affect performance. Some of the symptoms, however, can be very serious and will not only affect performance, but can also threaten health.
Serious symptoms
Among the reported deleterious manifestations of strenuous exercise are mucosal erosions and ischemic colitis, both observed after long distance running (Heer et al., 1987; Choi et al., 2001; Moses, 2005). For example, marathon runners and long distance triathletes occasionally have blood loss in feces in the hours following a marathon. Schaub et al. (1985) observed epithelial surface changes known to occur during ischemia upon colonoscopic inspection of one such triathlete following a marathon and suggested that ischemia of the lower GI tract induced the problems (Schaub et al., 1985). Blood loss as a result of ischemic colitis is not uncommon in athletes and can be profound in extreme cases. Proximal, distal or pancolitis, and even small bowel infarction have been reported in athletes and in some cases required surgery (Heer et al., 1987; Lucas & Schroy, 1998). Despite the high prevalence of symptoms, mild or severe, the etiology of these GI complaints in endurance athletes is still incompletely understood.
Causes of GI problems
While it is recognized that the etiology of exercise-induced GI distress is multifactorial, GI ischemia is often acknowledged as the main pathophysiological mechanism for the emergence of the symptoms (Ter Steege et al., 2008; De Oliveira & Burini, 2011; Ter Steege et al., 2011). The other factors are mechanical and nutritional in nature. Below we will first discuss the physiological effects of exercise that may contribute to the developments of GI symptoms.
Splanchnic hypoperfusion
There is large heterogeneity in the response of the GI system to exercise. Splanchnic hypoperfusion (reduced blood flow) during exercise ranges from mild circulatory changes to profound GI ischemia (Van Wijck et al., 2012). The consequences of hypoperfusion within the GI tract, i.e., epithelial injury and changes in GI permeability and epithelial barrier function, also differ greatly among individuals. The presence and nature of abdominal symptoms experienced by athletes vary from mild, exercise-related discomfort to severe ischemic colitis and diarrhea (Moses, 1990). During strenuous physical activity or exercise, norepinephrine is released from nerve endings and upon binding to α-adrenoreceptors of the sympathetic nervous system induces splanchnic vasoconstriction. This will result in an increase in the total splanchnic vascular resistance (Otte et al., 2005; Wright et al., 2011), while at the same time the vascular resistance in other tissues with increased activity during exercise (heart, lungs, active muscle, skin) is decreased (Otte et al., 2001; Qamar & Read, 1987). During maximal exercise, splanchnic blood flow may be reduced by up to 80% to provide sufficient blood flow to working muscle and skin. As blood is shunted from viscera to the active tissues (Qamar & Read, 1987), gut mucosal ischemia may result as well as increase the mucosa permeability (Casey et al., 2005). This in turn may be linked to nausea, vomiting, abdominal pain and diarrhea (De Oliveira & Burini, 2009; 2011), although convincing evidence for this is lacking (Ter Steege & Kolkman, 2012).
Changes in motility
Changes in motility might be observed at different levels of the intestinal tract, including the esophagus, stomach and intestine. Decreases in esophageal peristaltic activity and lower esophageal sphincter tone and increased transient lower sphincter relaxation have been observed and could be linked to gastroesophageal reflux during exercise (Peters et al., 2000). Gastric emptying may also be affected by exercise, although this probably only happens at high intensities of exercise or during intermittent activity (Leiper et al., 2001). Studies performed so far suggest that the effects of exercise on the small bowel as well as the colon are limited.
Absorption and gut permeability
Studies also suggest that there is little effect of exercise on intestinal absorption of both water and carbohydrate (Lambert et al., 1997; Ryan et al., 1998). It must be noted, however, that the studies used exercise intensities that were moderate and durations of exercise that were no longer than two hours. It is feasible that during higher exercise intensities, when blood flow to the intestine is compromised, and also after more prolonged exercise, that absorption is reduced. Oktedalen et al. (1992) reported increased intestinal permeability after a marathon, indicating damage to the gut and impaired gut function. There are numerous techniques available to study gut permeability but to date we have limited data. The information that is available suggests that gut permeability can be compromised in athletes (Pals et al., 1997). Although this has not been conclusively linked to gastrointestinal symptoms, one study showed that gut permeability in symptomatic runners was greater than in asymptomatic runners (Van Nieuwenhoven et al., 2004). On the other hand, in one long distance triathlon in extreme conditions where GI symptoms were highly prevalent, no compromised gut barrier function was observed as measured by bacterial translocation (LPS), a marker of mucosal damage and invasion of gram-negative intestinal bacteria and/or their toxic constituents (endotoxins) into the blood circulation (Jeukendrup et al., 2000). More research needs to be conducted before we have a clear understanding of the causes of GI distress.
Mechanical causes
The mechanical causes of GI problems are either impact-related or related to posture. For example, symptoms are more common in runners than in cyclists. This is thought to be a result of the repetitive high-impact mechanics of running and subsequent damage to the intestinal lining. This repetitive gastric jostling is also thought to contribute to lower GI symptoms such as flatulence, diarrhea and urgency. The mechanical trauma suffered by the gut from the repetitive impact of running, in combination with gut ischemia, likely account for GI bleeding. Posture can also have an effect on GI symptoms. For example on a bicycle, upper GI symptoms are more prevalent possibly due to increased pressure on the abdomen as a result of the cycling position, specifically when in the “aero” position. “Swallowing” air as a result of increased respiration and drinking from water bottles can result in mild to moderate stomach distress. In general, the only way to reduce the effects of these mechanical causes is by training.
Nutritional causes
It is known that nutrition can have a strong influence on GI distress, although many of the problems can persist in the absence of any food intake prior to or during exercise. Fiber, fat, protein and fructose have all been associated with a greater risk to develop GI symptoms. Dehydration, possibly as a result of inadequate fluid intake to offset sweating, may also exacerbate the symptoms. A study by Rehrer et al. (1992), demonstrated a link between nutritional practices and GI complaints during a half-Ironman distance triathlon. Gastrointestinal problems were more likely to occur with the ingestion of fiber, fat, protein and concentrated carbohydrate solutions during the triathlon. Beverages with high osmolalities (>500 mOsm/L) seemed to be associated with increased symptoms. The intake of dairy products may also be linked to the occurrence of gastrointestinal distress (De Vrese et al., 2001). Mild lactose intolerance is fairly common and could result in increased bowel activity and mild diarrhea. To minimize GI distress, all these risk factors must be taken into account, and milk products, fiber, high fat and high protein must be avoided 24 hours before competition and during exercise.
“Training the gut”
It has been shown that athletes who are not accustomed to fluid and food ingestion during exercise had a two-fold risk of developing GI symptoms compared with athletes who were accustomed to taking fluid and food during exercise (Ter Steege et al., 2008). The gut is highly adaptable and endurance athletes should incorporate nutritional training into their training plans (Jeukendrup & Mclaughlin, 2011). This was nicely demonstrated in a study by Cox et al. (2010). In this study, 16 endurance-trained cyclists or triathletes were pair-matched and randomly allocated to either a high-carbohydrate group (High group; n = 8) or an energy-matched low-carbohydrate group (Low group; n = 8) for 28 days. It became apparent after 28 days that the High group had higher exogenous carbohydrate oxidation rates during exercise than the Low group (Cox et al., 2010), a finding that was attributed to improved absorption. The training would have reduced the chances of GI distress as improved intestinal absorption is generally associated with improved tolerance of fluids and foods during exercise (Jeukendrup & Mclaughlin, 2011).
Other contributing factors
It has been reported that large numbers of athletes use analgesics to relieve existing or anticipated pain (Gorski et al., 2011). The use of non-selective non-steroidal anti-inflammatory drugs (NSAIDs) has been associated with a three- to five-fold increased risk of upper GI complications, mucosal bleeding or perforation compared to no medication (Gabriel et al., 1991).
Prevention of gastrointestinal problems
In order to prevent GI distress, a few guidelines can be provided. It must be noted, however, that these are based on limited research. Nevertheless, anecdotally these guidelines seem to be effective:
Avoid milk products that contain lactose as even mild lactose intolerance can cause problems during exercise. For instance it is possible to avoid milk completely or get lactose-free milk. Soy, rice and almond milks generally don’t contain lactose.
Avoid high-fiber foods in the day or even days before competition. For the athlete in training, a diet with adequate fiber will help to keep the bowel regular. Fiber before race day is different. By definition, fiber is not digestible, so any fiber that is eaten essentially passes through the intestinal tract. Increased bowel movements during exercise are not desirable and will accelerate fluid loss. It may also result in unnecessary gas production which might cause cramping. A low-fiber diet the day before (or even a couple of days before) is recommended, especially for those individuals who are prone to develop GI symptoms. Choose processed white foods, like regular pasta, white rice and plain bagels instead of whole grain bread, high-fiber cereals and brown rice. Check the food labels for fiber content. Most fruits and vegetables are high in fiber but there are a few exceptions as zucchini, tomatoes, olives, grapes and grapefruit all have less than one gram of fiber per serving.
Avoid aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen. Both aspirin and NSAIDs have been shown to increase intestinal permeability and may increase the incidence of GI complaints. The use of NSAIDs in the pre-race period should be discouraged.
Avoid high-fructose foods (in particular drinks that have exclusively fructose). Interestingly, however, fructose in combination with glucose may not cause problems and may even be better tolerated.
Avoid dehydration since it can exacerbate GI symptoms. Start the race (or training) well hydrated.
Practice new nutrition strategies by experimenting with your pre-race and race-day nutrition plan many times prior to race day. This will allow the athlete to determine what works and what does not work and also will reduce the chances of getting GI symptoms.
CONCLUSION
The gut is an important athletic organ because it is responsible for the delivery of water and nutrients during exercise. Both upper- and lower-gastrointestinal (GI) complaints are highly prevalent among athletes during exercise (especially endurance athletes) and can negatively impact performance. In severe cases it can pose health risks too. Most GI complaints during exercise are mild and of no risk to health, but hemorrhagic gastritis, hematochezia and ischemic bowel can present serious medical challenges. Nutritional training and appropriate nutritional choices can reduce the risk of GI discomfort during exercise by assuring rapid gastric emptying and absorption of water and nutrients and by maintaining adequate perfusion of the splanchnic vasculature.
References
Casey, E., Mistry, D. J. and Macknight, J. M. (2005). Training room management of medical conditions: sports gastroenterology. Clin Sports Med. 24:525-40, viii.
Choi, S. C., Choi, S. J., Kim, J. A., Kim, T. H., Nah, Y. H., Yazaki, E. and Evans, D. F. (2001). The role of gastrointestinal endoscopy in long-distance runners with gastrointestinal symptoms. Eur J Gastroenterol Hepatol. 13:1089-94.
Cox, G. R., Clark, S. A., Cox, A. J., Halson, S. L., Hargreaves, M., Hawley, J. A., Jeacocke, N., Snow, R. J., Yeo, W. K. and Burke, L. M. (2010). Daily training with high carbohydrate availability increases exogenous carbohydrate oxidation during endurance cycling. J Appl Physiol. 109:126-34.
De Oliveira, E. P. and Burini, R. C. (2009). The impact of physical exercise on the gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 12:533-8.
De Oliveira, E. P. and Burini, R. C. (2011). Food-dependent, exercise-induced gastrointestinal distress. J Int Soc Sports Nutr. 8:12.
De Vrese, M., Stegelmann, A., Richter, B., Fenselau, S., Laue, C. and Schrezenmeir, J. (2001). Probiotics--compensation for lactase insufficiency. Am J Clin Nutr. 73:421S-429S.
Gabriel, S. E., Jaakkimainen, L. and Bombardier, C. (1991). Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 115:787-96.
Gorski, T., Cadore, E. L., Pinto, S. S., Da Silva, E. M., Correa, C. S., Beltrami, F. G. and Kruel, L. F. (2011). Use of NSAIDs in triathletes: prevalence, level of awareness and reasons for use. Br J Sports Med. 45:85-90.
Heer, M., Repond, F., Hany, A., Sulser, H., Kehl, O. and Jager, K. (1987). Acute ischaemic colitis in a female long distance runner. Gut. 28:896-9.
Jeukendrup, A. E. and Mclaughlin, J. (2011). Carbohydrate ingestion during exercise: effects on performance, training adaptations and trainability of the gut. Nestle Nutr Inst Workshop Ser. 69:1-12; discussion 13-7.
Jeukendrup, A. E., Vet-Joop, K., Sturk, A., Stegen, J. H., Senden, J., Saris, W. H. and Wagenmakers, A. J. (2000). Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long- distance triathlon in highly trained men. Clin Sci (Colch). 98:47-55.
Lambert, G. P., Chang, R. T., Xia, T., Summers, R. W. and Gisolfi, C. V. (1997). Absorption from different intestinal segments during exercise. J Appl Physiol. 83:204-12.
Leiper, J. B., Prentice, A. S., Wrightson, C. and Maughan, R. J. (2001). Gastric emptying of a carbohydrate-electrolyte drink during a soccer match. Med Sci Sports Exerc. 33:1932-8.
Lucas, W. and Schroy, P. C., 3rd (1998). Reversible ischemic colitis in a high endurance athlete. Am J Gastroenterol. 93:2231-4.
Moses, F. M. (1990). The effect of exercise on the gastrointestinal tract. Sports Med. 9:159-72.
Moses, F. M. (2005). Exercise-associated intestinal ischemia. Curr Sports Med Rep. 4:91-5.
Oktedalen, O., Lunde, O. C., Opstad, P. K., Aabakken, L. and Kvernebo, K. (1992). Changes in the gastrointestinal mucosa after long-distance running. Scand J Gastroenterol. 27:270-4.
Otte, J. A., Geelkerken, R. H., Oostveen, E., Mensink, P. B., Huisman, A. B. and Kolkman, J. J. (2005). Clinical impact of gastric exercise tonometry on diagnosis and management of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol. 3:660-6.
Otte, J. A., Oostveen, E., Geelkerken, R. H., Groeneveld, A. B. and Kolkman, J. J. (2001). Exercise induces gastric ischemia in healthy volunteers: a tonometry study. J Appl Physiol. 91:866-71.
Pals, K. L., Chang, R. T., Ryan, A. J. and Gisolfi, C. V. (1997). Effect of running intensity on intestinal permeability. J Appl Physiol. 82:571-6.
Peters, H. P., Bos, M., Seebregts, L., Akkermans, L. M., Van Berge Henegouwen, G. P., Bol, E., Mosterd, W. L. and De Vries, W. R. (1999). Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: prevalence, medication, and etiology. Am J Gastroenterol. 94:1570-81.
Peters, H. P., Wiersma, J. W., Koerselman, J., Akkermans, L. M., Bol, E., Mosterd, W. L. and De Vries, W. R. (2000). The effect of a sports drink on gastroesophageal reflux during a run-bike-run test. Int J Sports Med. 21:65-70.
Pfeiffer, B., Cotterill, A., Grathwohl, D., Stellingwerff, T. and Jeukendrup, A. E. (2009). The effect of carbohydrate gels on gastrointestinal tolerance during a 16-km run. Int J Sport Nutr Exerc Metab. 19:485-503.
Pfeiffer, B., Stellingwerff, T., Hodgson, A. B., Randell, R., Poettgen, K., Res, P. and Jeukendrup, A. E. (2011). Nutritional intake and gastrointestinal problems during competitive endurance events. Med Sci Sports Exerc. In review.
Qamar, M. I. and Read, A. E. (1987). Effects of exercise on mesenteric blood flow in man. Gut. 28:583-7.
Rehrer, N. J., Van Kemenade, M., Meester, W., Brouns, F. and Saris, W. H. M. (1992). Gastrointestinal complaints in relation to dietary intake in triathletes. Int J Sport Nutr. 2:48-59.
Ryan, A. J., Lambert, G. P., Shi, X., Chang, R. T., Summers, R. W. and Gisolfi, C. V. (1998). Effect of hypohydration on gastric emptying and intestinal absorption during exercise. J Appl Physiol. 84:1581-8.
Schaub, N., Spichtin, H. P. and Stalder, G. A. (1985). [Ischemic colitis as a cause of intestinal bleeding after marathon running]. Schweiz Med Wochenschr. 115:454-7.
Ter Steege, R. W., Geelkerken, R. H., Huisman, A. B. and Kolkman, J. J. (2011). Abdominal symptoms during physical exercise and the role of gastrointestinal ischaemia: a study in 12 symptomatic athletes. Br J Sports Med.
Ter Steege, R. W. and Kolkman, J. J. (2012). Review article: the pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment Pharmacol Ther. 35:516-28.
Ter Steege, R. W., Van Der Palen, J. and Kolkman, J. J. (2008). Prevalence of gastrointestinal complaints in runners competing in a long-distance run: an internet-based observational study in 1281 subjects. Scand J Gastroenterol. 43:1477-82.
Van Nieuwenhoven, M. A., Brouns, F. and Brummer, R. J. (2004). Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol. 91:429-34.
Van Wijck, K., Lenaerts, K., Grootjans, J., Wijnands, K. A., Poeze, M., Van Loon, L. J., Dejong, C. H. and Buurman, W. A. (2012). Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 303:G155-68.
Wright, H., Collins, M., Villiers, R. D. and Schwellnus, M. P. (2011). Are splanchnic hemodynamics related to the development of gastrointestinal symptoms in ironman triathletes? A prospective cohort study. Clin J Sport Med. 21:337-43.