KEY POINTS
- Branched-chain amino acids (BCAA) are essential amino acids (EAA) that play several important roles in muscle metabolism.
- The BCAA – especially leucine – are critical for stimulation of molecular signaling that leads to muscle protein synthesis and muscle protein breakdown. However, there is no research that has assessed the response of muscle protein synthesis to BCAA (or leucine) ingestion alone (without any other nutrients and/or not as part of an intact protein) following exercise.
- Ingestion of BCAA without co-ingestion of a source of the other EAA will not stimulate a maximal muscle protein synthesis response. While BCAA ingestion (primarily leucine) stimulates the molecular anabolic signaling pathways involved in muscle protein synthesis, the response, particularly after exercise, will be limited with only the endogenous amino acids available as substrate.
- There is preliminary evidence that co-ingestion of carbohydrate and fat with smaller doses of EAA may enhance muscle protein synthesis following exercise. However, a more systematic evaluation of this finding needs to be performed before solid recommendations can be made.
- There is insufficient evidence to indicate that BCAA ingestion is effective for stimulating muscle protein synthesis following endurance exercise.
- There is evidence that BCAA inhibit muscle protein breakdown in cell culture studies, rodent muscle and human muscle at rest. However, there is no evidence that BCAA ingestion inhibits post-exercise muscle protein breakdown in humans.
- It is not clear that a nutritional intervention, including BCAA ingestion, aimed at inhibiting post-exercise muscle protein breakdown is a positive goal.
- The evidence for use of BCAA as a countermeasure to reduce exercise-induced muscle damage is, at best, equivocal and not as clear as touted by many sources.
INTRODUCTION
The branched-chain amino acids (BCAA), isoleucine, leucine and valine, are essential amino acids (EAA) that have received a great deal of attention recently in sports and exercise nutrition recommendations, particularly in the context of enhancing the response of muscle to exercise. Over the past few years supplementation with BCAA has become a popular component of nutritional support for intense exercise and training, based on the claim that BCAA enhance recovery and increase adaptations to training. For example, a Google search for “BCAA and recovery” yields over 750,000 hits. The claims for these products are based on a wide range of mechanisms: from enhanced muscle protein synthesis (MPS) and decreased muscle protein breakdown (MPB) to protection of the immune system, increased fat oxidation and decreased muscle soreness, among many others. The physiological rationale for these claims, let alone robust evidence from well-controlled human studies, is often weak, if not completely lacking.
The aspect of recovery that is most commonly attributed to BCAA supplementation is muscle building or anabolism, of which MPS and MPB are the metabolic processes that have the most influence. The net balance between the synthesis and breakdown (NBAL) of any particular protein determines the amount of that protein in muscle and influences the function of that protein. For example, increases in muscle size and strength stem from increases in the NBAL of myofibrillar proteins. The anabolic response to exercise is often considered to be synonymous with muscle hypertrophy. However, muscle anabolism is also important for repair and remodeling of tissue leading to enhanced recovery and adaptation to exercise training of different types. Following resistance exercise, muscle anabolism leads to repair of damaged muscle cells and tissue and muscle growth. Following endurance exercise, muscle repair and adaptations leading to enhanced aerobic power and endurance performance result from enhanced anabolic processes. Both aspects of recovery have been investigated in the context of BCAA supplementation.
Branched-chain amino acids have long been known to influence both MPS and MPB. Early preclinical work (animal and cell culture) clearly indicated that BCAA had a profound influence on both MPS and MPB (Buse & Reid, 1975). Subsequent study of muscle in resting humans showed that leucine influences MPS (Louard et al., 1990). Amino acids provide substrate for the synthesis of new proteins, but BCAA and especially leucine have also been shown to act as specific signals that stimulate the initiation of translation – the process that leads to MPS (Kimball & Jefferson, 2006). Thus, BCAA supplementation may act as a stimulus for MPS, as well as providing substrate when needed. Moreover, the influence of BCAA on MPB (Louard et al., 1995; Nair et al., 1992) provides a strong rationale for supplementation following exercise to enhance the balance between the synthesis and breakdown of proteins that are important for optimization of recovery, enhanced adaptation to training and/or muscle hypertrophy.
It seems clear that a case can be made for the use of BCAA supplementation with intense exercise. Moreover, many people clearly believe that supplementation of these amino acids is an effective measure to enhance recovery and adaptation to training. The purpose of this article is to examine the evidence for the use of BCAA supplementation in the context of enhancing muscle anabolism. The primary focus will be on the response of MPS and MPB, as well as the molecular signaling pathways associated with those metabolic processes, to BCAA supplementation during and following exercise.
A COMMENT ON SUPPLEMENTATION MODELS
The responses of MPS and MPB to BCAA supplementation in association with exercise have been examined using several models of supplementation. Studies have examined the response of either MPS and/or MPB to the ingestion of leucine alone or in combination with the other BCAA (valine and isoleucine), all other EAA, or with intact proteins. The combination of nutrients with which BCAA are administered has implications for how the results of the studies should be interpreted and for the conclusions drawn. Since leucine is commonly thought to be the most influential BCAA (Kimball & Jefferson, 2006), most studies have centered on the response to leucine ingestion. Naturally, the measured responses may also vary depending on the particular circumstances in which the BCAA are ingested. Supplements have been ingested prior to, during and following exercise in various studies. The varied nature of the studies has complicated the interpretations and the ability to make solid conclusions about the topic. Nevertheless, given that these supplements are widely used, it is worthwhile to examine the available information and attempt to provide some practical recommendations given the limitations of the methodologies and study designs.
MUSCLE PROTEIN SYNTHESIS AND BREAKDOWN
The influence of BCAA supplementation on muscle hypertrophy is attributed primarily to the stimulation of the molecular pathways leading to MPS by leucine (Kimball & Jefferson, 2006). It is well accepted that leucine and the other BCAA stimulate the mammalian target of rapamycin complex (mTORC)-signaling pathway – the molecular pathway leading to initiation of the translational process – both in cell culture studies (Kimball & Jefferson, 2006) and in vivo in rodents and humans (Bolster et al., 2004). Increasing leucine, and possibly isoleucine and valine, concentrations in the blood by ingestion of a source of BCAA stimulates the mTORC-signaling pathway leading to increased MPS (Wilkinson et al., 2013). The “leucine trigger” theory was developed to describe the importance of increasing leucine levels. This theory suggests that leucine must reach a threshold to maximally stimulate the anabolic processes in muscle. This theory predicts that the source of leucine that leads to the leucine “trigger point” the fastest provides the most potent anabolic stimulation (Breen & Phillips, 2012). Thus, BCAA supplementation has been advocated as an important component of any diet to maximize muscle hypertrophy with exercise.
Branched-chain amino acid supplementation and molecular anabolic signaling
Resistance exercise itself is a potent stimulator of mTORC signaling. An excellent summary of the molecular responses to resistance and endurance exercise can be found in a recent SSE written by Dr. Keith Baar [SSE 136]. Anabolic signaling, particularly the activation of the protein, p70s6 kinase, is associated with MPS and muscle growth in both rats and humans (Baar & Esser, 1999) following resistance exercise training. Thus, the question that we must consider related to muscle growth and BCAA is whether BCAA supplementation enhances the response of the mTORC-signaling pathway in addition to the stimulation the pathway receives from resistance exercise.
Investigations examining the response of muscle anabolic signaling to exercise and BCAA supplementation demonstrated that BCAA and resistance exercise stimulate signaling through the mTORC pathway, assessed primarily by phosphorylation of p70s6 kinase, in both combined and independent manners (Apro & Blomstrand, 2010; Karlsson et al., 2004). Consistent with earlier work in cells and animals (Kimball & Jefferson, 2006), the most important component of the BCAA supplements appeared to be leucine. However, administration of an EAA solution, including isoleucine and valine, but with no leucine, led to a limited response of mTORC-signaling compared to the response to ingesting a solution including leucine (Moberg et al., 2014). Nevertheless, it should be noted that mTORC-signaling was increased, even if somewhat limited, following EAA ingestion without leucine (Moberg et al., 2014). Thus, leucine is important, but not absolutely necessary, to garner at least some anabolic signaling response in muscle after resistance exercise, i.e., providing the other EAA are sufficient, even if not optimal. The evidence clearly indicates that BCAA supplementation enhances the anabolic response of the molecular signaling pathways leading to MPS following resistance exercise.
Branched-chain amino acid supplementation and muscle protein synthesis
Despite ample evidence that BCAA supplementation has an anabolic impact on skeletal muscle, at least on a molecular level, the response of MPS to BCAA supplementation after exercise is not so clear. Certainly, ingestion of EAA, including leucine and the other BCAA, is critical for the response of MPS following exercise (Borsheim et al., 2002; Tipton et al., 1999). Ingestion of leucine alone stimulates MPS in resting human muscle (Wilkinson et al., 2013). Moreover, MPS that is reduced following exercise in rats is restored by ingestion of leucine (Anthony et al., 1999). Thus, there is ample indirect evidence to predict that BCAA supplementation following exercise should increase MPS. Strangely, despite a great deal of interest on all levels, a direct measurement of MPS in response to supplementation of BCAA or leucine alone (without the other EAA), following resistance exercise in humans has yet to be published.
The impact of BCAA ingestion on MPS following exercise has been investigated using a variety of methods of amino acid ingestion. Unfortunately, given the disparate models used, the results can only be considered equivocal. Several authors investigated the impact of the addition of leucine to protein or EAA solutions on MPS following resistance exercise. It is clear that EAA are critical for the enhanced metabolic response of muscle following resistance exercise (Tipton et al., 1999). However, adding leucine (or BCAA) to EAA or protein does not necessarily enhance the response. Previously, we demonstrated that the addition of leucine to protein did not increase NBAL following exercise (Tipton et al., 2009), but MPS was not directly measured. Similarly, Koopman et al. (2005) found no increase in MPS following resistance exercise with the addition of leucine to protein. The amount of protein ingested in these studies may be crucial to the responses that were measured. The volunteers in both studies ingested a relatively large amount of protein (0.2 g/kg/h protein hydrolysate with or without 0.1 g/kg leucine (Koopman et al., 2005) and 16.6 g of whey protein with 3.4 g leucine (Tipton et al., 2009). Thus, it seems likely that when sufficient protein/EAA is ingested to provide optimal leucine availability (above the “leucine trigger” level), then additional leucine does not enhance the response. This interpretation is likely an oversimplification and a more systematic investigation of this concept is necessary before solid recommendations may be made.
Another important factor to consider in relation to BCAA supplementation is the availability of EAA as substrate for MPS. Providing additional leucine may not be sufficient to maximally stimulate MPS following exercise when lower amounts of EAA, in free form or as part of an intact protein, are simultaneously ingested. Researchers at McMaster University in Canada investigated the importance of leucine for stimulation of MPS with varying amounts of protein. Previously, it had been established that 25 g of whey protein might provide an optimal dose for stimulation of MPS at rest and following exercise (Churchward-Venne et al., 2012; Moore et al., 2008). Thus, the Canadian scientists, led by Professor Stuart Phillips, compared the response of MPS to ingestion of 25 g of whey protein to the response with ingestion of 6.25 g of whey protein. However, leucine ingestion was equated by adding leucine to the lower protein dose. They reported that ingestion of leucine plus a “suboptimal” dose (6.25 g) of whey protein resulted in rates of MPS similar to those after ingestion of 25 g (an optimal dose) of whey protein at rest (Churchward-Venne et al., 2012). However, following resistance exercise, rates of MPS were greater when 25 g of whey protein were ingested than when 6.25 g of whey protein were ingested with additional leucine. Notwithstanding the equivalent amount of leucine ingested, the response of MPS following exercise was less with a suboptimal dose of whey protein compared to an optimal dose. It is likely that the enhanced ability of the muscle to utilize ingested protein following exercise (Witard et al., 2014) meant that EAA availability was limited with the lower dose of whey protein. These results suggest that availability of EAA may be a factor that is critical for the optimal response of MPS following exercise.
The supplementation of BCAA (leucine) may not be ideal if the response of MPS is limited by the lack of EAA availability. Even given a maximal stimulation of the anabolic signaling pathways with high doses of leucine (Apro & Blomstrand, 2010), sufficient substrate must be present to allow for the optimal rates of MPS. The difference in MPS between resting and contracted muscle with low protein (6.25 g) plus leucine and higher protein (25 g) ingestion supports this interpretation (Churchward-Venne et al., 2012). With the greater stimulation of the synthetic machinery by exercise, more substrate is necessary to sustain the maximal rates of MPS and these rates decline after a short time as reported by Churchward-Venne et al. (2012). Thus, it seems clear that ingestion of an intact protein is preferable to ingestion of smaller doses of protein or other sources of amino acids if the total amount of EAA is not sufficient to sustain MPS following exercise.
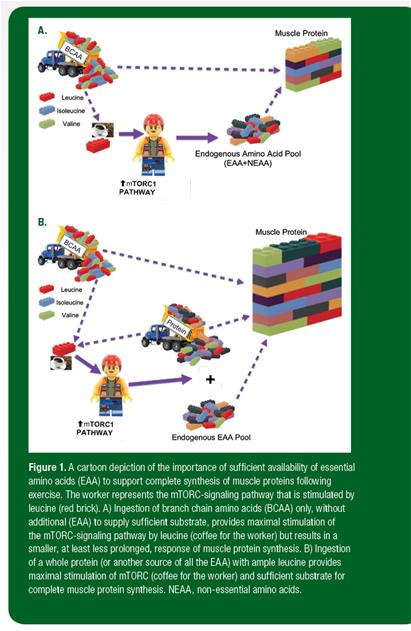
This metabolic situation may be thought of as analogous to building a wall (Figure 1). In this analogy, muscle protein may be thought of as the wall and amino acids as the bricks necessary to build it. Each type of wall requires a particular combination of bricks. Certain types of bricks (nonessential amino acids) are always lying about and available. However, the wall can only be completed if other bricks are made available from outside (EAA from dietary protein). The mTORC signaling pathway may be thought of as the worker that puts the bricks together to form the wall. Leucine is a special brick: It is necessary as part of the wall, but it also comes with a pot of coffee that can stimulate the worker into faster and more effective wall building. Therefore, if BCAA are ingested, the leucine (coffee) stimulates the worker into maximum activity and the available amino acids, both EAA and nonessential amino acids, are used to begin wall building. However, despite the willingness and energy of the worker, at some point, the worker will run short of some of the necessary bricks (EAA). Subsequently, wall building will slow and eventually cease. On the other hand, when a complete protein or another source of all the amino acids is consumed, all the necessary bricks to build the wall are available to the worker, the leucine brick gets the worker going, and the largest wall is completed.
Branched-chain amino acid supplementation and muscle protein synthesis with co-ingestion of other macronutrients
The relationship of protein and amino acid ingestion to MPS may be altered by ingestion of other nutrients along with the amino acid source. A follow-up study by the same previously mentioned Canadian researchers also showed that MPS in response to ingestion of 25 g of whey protein following exercise was greater than the response to a low dose (6.25 g) of whey protein plus additional leucine when ingested as part of a mixed macronutrient (including carbohydrate and fat) beverage (Churchward-Venne et al., 2014). However, the ingestion of a higher dose of leucine added to 6.25 g of whey protein resulted in similar rates of MPS to the 25 g of whey protein ingestion in this situation. So, protein and amino acids consumed in the context of a mixed meal in the more recent study (Churchward-Venne et al., 2014), appeared to produce a differential response compared to that when consumed in isolation as in the former study (Churchward–Venne et al., 2012). These results are supported by a recent investigation. In both older and young adults, the addition of free leucine to a drink containing 10 g protein and 24 g carbohydrate enhanced the response of MPS following resistance exercise (Atherton et al., 2016). Taken together, these studies suggest that a suboptimal dose of protein consumed with a high dose of leucine can “rescue” the rates of MPS after exercise to levels similar to optimal protein doses, but seemingly only within the context of consumption with other macronutrients.
Branched-chain amino acid supplementation and muscle protein breakdown
The potential impact of BCAA supplementation on muscle growth and recovery from exercise may be related to a role in the process of MPB in addition to MPS. Thus, BCAA supplementation may affect both sides of the NBAL equation. Early studies demonstrated that BCAA reduced whole body protein breakdown (Ferrando et al., 1995), as well as MPB (Louard et al., 1995; Nair et al., 1992) at rest. The impact of BCAA on MPB may be mediated by the leucine metabolite, β-hydroxy-β-methylbutyrate (Wilkinson et al., 2013). However, to date no study has investigated the impact of BCAA supplementation on MPB following exercise. Thus, whereas there are a multitude of claims regarding the importance of BCAA supplementation for the reduction of MPB with exercise, the effectiveness of BCAA supplementation for enhancing NBAL following exercise via reduced MPB is unsubstantiated.
Another consideration for the impact of BCAA, or any other nutrients, on MPB relates to whether it is desirable to reduce MPB during or following exercise. The initial, perhaps even intuitive, answer is a resounding yes with the reasoning that if MPB is limited, then NBAL is increased and muscles get larger and stronger. However, that interpretation is probably far too simplistic: MPB is not merely a component of NBAL impacting muscle hypertrophy. Rather, MPB is also a key component of muscle protein turnover leading to remodeling and reconditioning of muscle. So, in some circumstances, elevated MPB may be advantageous – for example, to degrade and remove damaged proteins. Moreover, degradation of proteins that are maladaptive and/or not used to provide amino acids for synthesis of new proteins during adaptation may be critical. None of the methods currently available to measure MPB permit identification of the proteins that are being degraded. So, if reduced MPB is measured in response to BCAA, or any other nutrient, it is not clear if the proteins being broken down are myofibrillar proteins, thus reducing hypertrophy, or other proteins. Therefore, even if there was solid evidence that BCAA supplementation reduced MPB during and following exercise – and there is not – it still would not be clear that the reduced MPB is a positive factor for optimal recovery from exercise and adaptation to training.
Branched-chain amino acid supplementation for muscle protein synthesis following endurance exercise
Whereas a number of studies have focused on the influence of BCAA supplementation on MPS following resistance exercise, the impact on MPS following endurance exercise has received less attention. To date, there are only two studies that have investigated this question, with many differences in design between the studies. In the earlier study, doubling the amount of leucine in an EAA solution increased the rate of MPS following cycling (Pasiakos et al., 2011). In the more recent study, whereas ingestion of protein with additional leucine following exercise increased MPS, tripling the amount of protein and leucine did not result in further stimulation (Rowlands et al., 2015). Nevertheless, both of these studies, taken together, are consistent with the notion proposed earlier. That is, if insufficient leucine is ingested, then further stimulation of molecular pathways by additional leucine results in increased MPS (Pasiakos et al., 2011). However, when sufficient leucine is ingested, additional leucine does not further enhance MPS following exercise (Rowlands et al., 2015). Caution must be urged before any solid recommendations are made based on just two studies. Differences in the subjects studied, co-ingestion of other nutrients, amounts of BCAA ingested, ingestion of free EAA or intact protein may all impact the results. Thus, at least at this juncture, it is perhaps premature to try to make any solid conclusions regarding the impact of BCAA ingestion on MPS following endurance exercise.
The physiological relevance of increased MPS in response to BCAA ingestion following endurance exercise is less clear than following resistance exercise. Previously, we demonstrated that protein ingestion, including relatively high amounts of BCAA, following endurance exercise resulted in increased myofibrillar MPS (Breen et al., 2011). The increase in MPS following exercise is often associated primarily with muscle hypertrophy – perhaps erroneously in many cases (Mitchell et al., 2014). However, increased myofibrillar MPS following endurance exercise may be associated more with muscle remodeling and reconditioning leading to enhanced recovery from the exercise, an argument we put forward in Breen et al. (2011). Thus, at this point at least, it is not clear what, if any, importance can be ascribed to BCAA ingestion after endurance exercise.
Branched-chain amino acid supplementation and muscle damage
There is some evidence to support the claim that BCAA supplementation is an effective treatment to improve recovery from exercise-induced muscle damage (Howatson et al., 2012; Jackman et al., 2010). However, not all studies support such claims (Areces et al., 2014; Foure et al., 2016), thus the data can be considered at best, equivocal. Moreover, the studies that demonstrated a statistically significant decrease in muscle soreness following exercise with BCAA ingestion were not able to identify a mechanism (Howatson et al., 2012; Jackman et al., 2010). Increased MPS is often claimed to be the mechanism behind any amelioration of muscle damage with exercise and BCAA ingestion. However, given the slow turnover rate of any damaged proteins that may be involved, this mechanism is difficult to accept and claims of this nature do not have a solid physiological basis. Finally, many of the models that are used to study muscle damage are not ecologically valid and the degree of any improvement noted is often rather minor even if statistically significant (Jackman et al., 2010). Thus, recommendations for BCAA supplementation to counter muscle damaging-inducing exercise cannot be supported, at least based on a full and objective assessment of the evidence.
SUMMARY AND PRACTICAL APPLICATIONS
The supplementation of BCAA has many purported benefits for athletes and other exercisers. There is some evidence for everything from increasing MPS, decreasing MPB, and increasing fat oxidation (not discussed in this SSE) to decreasing muscle soreness and enhancing the immune response to exercise (also not discussed in this SSE). However, the current evidence does not stand up to critical scrutiny. There is no question that BCAA supplementation, due to the leucine content, stimulates the molecular pathways leading to enhanced MPS. However, it is not clear that BCAA supplements alone are effective for optimal stimulation of MPS following exercise. In fact, consuming BCAA without sufficient amounts of the other EAA will not produce the full MPS response. Supplementation with BCAA inhibit MPB at rest, but there is yet no evidence to suggest that they inhibit MPB leading to optimal NBAL after exercise. Furthermore, it is not clear that inhibition of global MPB after exercise is desirable for enhanced muscle hypertrophy, adaptation to training, or recovery from exercise. Finally, there is not enough information regarding BCAA ingestion and MPS following endurance exercise to make any conclusions and there is no reason to recommend BCAA supplementation during or following such exercise.
- Overall, based on the available evidence, the best nutritional recommendation to optimize adaptations to training, including muscle hypertrophy and enhanced oxidative metabolism, would still be to eat sufficient high-quality protein (that includes BCAA, of course) in the context of meals. Meals should be based, as much as possible, on whole, unprocessed foods and include lots of fresh fruits and vegetables.
- There is no reason to consume BCAA supplements for enhanced stimulation of MPS and/or decreased MPB. High-quality protein in foods or, if preferred for convenience, supplements (including for example, whey protein, egg protein, other dairy proteins, soy protein) should be consumed to provide sufficient BCAA and leucine, as well as the other EAA.
- There is preliminary, albeit insufficiently substantiated, evidence that consuming other macronutrients with protein or EAA supplements may enhance the response of MPS following exercise.
- There is insufficient evidence to support a recommendation to consume BCAA supplements to ameliorate muscle damage.
Figure 1. A cartoon depiction of the importance of sufficient availability of essential amino acids (EAA) to support complete synthesis of muscle proteins following exercise. The worker represents the mTORC-signaling pathway that is stimulated by leucine (red brick). A) Ingestion of branch chain amino acids (BCAA) only, without additional (EAA) to supply sufficient substrate, provides maximal stimulation of the mTORC-signaling pathway by leucine (coffee for the worker) but results in a smaller, at least less prolonged, response of muscle protein synthesis. B) Ingestion of a whole protein (or another source of all the EAA) with ample leucine provides maximal stimulation of mTORC (coffee for the worker) and sufficient substrate for complete muscle protein synthesis. NEAA, non-essential amino acids.
REFERENCES
Anthony, J.C., T.G. Anthony, and D.K. Layman (1999). Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J. Nutr. 129:1102-1106.
Apro, W., and E. Blomstrand (2010). Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol. 200:237-248.
Areces, F., J.J. Salinero, J. Abian-Vicen, C. González-Millán, C. Gallo-Salazar, D. Ruiz-Vicente, B. Lara, and J. Del Coso (2014). A 7-day oral supplementation with branched-chain amino acids was ineffective to prevent muscle damage during a marathon. Amino Acids. 46:1169-1176.
Atherton, P.J., K. Kumar, A.L. Selby, D. Rankin, W. Hildebrandt, B.E. Phillips, J.P. Williams, N. Hiscock, and K. Smith (2016). Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. E-pub ahead of print (pii: S0261-5614(16)30071-1).
Baar, K., and K. Esser (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am. J. Physiol. 276:C120-C127.
Bolster, D.R., L.S. Jefferson, and S.R. Kimball (2004). Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc. Nutr. Soc. 63:351-356.
Borsheim, E., K.D. Tipton, S.E. Wolf, and R.R. Wolfe (2002). Essential amino acids and muscle protein recovery from resistance exercise. Am. J. Physiol. 283:E648-E657.
Breen, L., A. Philp, O.C. Witard, S.R. Jackman, A. Selby, K. Smith, K. Baar, and K.D. Tipton (2011). The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J. Physiol. 589:4011-4025.
Breen, L., and S.M. Phillips (2012). Nutrient interaction for optimal protein anabolism in resistance exercise. Curr. Opin. Clin. Nutr. Metab. Care. 15:226-232.
Buse, M.G., and S.S. Reid (1975). Leucine. A possible regulator of protein turnover in muscle. J. Clin. Invest. 56:1250-1261.
Churchward-Venne, T.A., N.A. Burd, C.J. Mitchell, D.W. West, A. Philp, G.R. Marcotte, S.K. Baker, K. Baar, and S.M. Phillips (2012). Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 590:2751-2765
Churchward-Venne, T.A., L. Breen, D.M. Di Donato, A.J. Hector, C.J. Mitchell, D.R. Moore, T. Stellingwerff, D. Breuille, E.A. Offord, S.K. Baker, and S.M. Phillips (2014). Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am. J. Clin. Nutr. 99:276-286.
Ferrando, A,A,, B.D. Williams, C.A. Stuart, H.W. Lane, and R.R. Wolfe (1995). Oral branched-chain amino acids decrease whole-body proteolysis. J. Parenter. Enteral. Nutr. 19:47-54.
Foure, A., K. Nosaka, M. Gastaldi, J.-P. Matteia, H. Boudinete, M. Guyea, C. Vilmena, Y. Le Fura, D. Bendahan, and J. Gondin (2016). Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: A randomized placebo controlled trial. Clin. Nutr. 35:83-94.
Howatson, G., M. Hoad, S. Goodall, J. Tallent, P.G. Bell, and D.N. French (2012). Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 8:9, 20.
Jackman, S.R., O.C. Witard, A.E. Jeukendrup, and K.D. Tipton (2010). Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med. Sci. Sports Exerc. 42:962-970.
Karlsson, H.K., P.A. Nilsson, J. Nilsson, A.V. Chibalin, J.R. Zierath, and E. Blomstrand (2004). Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am. J. Physiol. 287:E1-E7.
Kimball, S.R., and L.S. Jefferson (2006). Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 136:227S-2231S.
Koopman, R., A.J. Wagenmakers, R.J. Manders, A.H. Zorenc, J.M. Senden, M. Gorselink, H.A. Keizer, and L.J. van Loon (2005). Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. 288:E645-E653.
Louard, R.J., E.J. Barrett, and R.A. Gelfand (1990). Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin. Sci. 79:457-466.
Louard, R.J., E.J. Barrett, and R.A. Gelfand (1995). Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism. 44:424-429.
Mitchell, C.J., T.A. Churchward-Venne, G. Parise, L. Bellamy, S.K. Baker, K. Smith, P.J. Atherton, and S.M. Phillips (2014). Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One. 9:e89431.
Moberg, M., W. Apro, I. Ohlsson, M. Ponten, A. Villanueva, B. Ekblom, and E. Blomstrand (2014). Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl. Physiol. Nutr. Metab. 39:183-194.
Moore, D.R., M.J. Robinson, J.L. Fry, J.E.Tang, E.I. Glover, S.B. Wilkinson, T. Prior, M.A. Tarnopolsky, and S.M. Phillips (2008). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89:161-168.
Nair, K.S., R.G. Schwartz, and S. Welle (1992). Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am. J. Physiol. 263:E928-E934.
Pasiakos, S.M., H.L. McClung, J.P. McClung, L.M. Margolis, N.E. Andersen, G.J. Cloutier, M.A. Pikosky, J.C. Rood, R.A. Fielding, and A.Y. Young (2011). Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am. J. Clin. Nutr. 94:809-818.
Rowlands, D.S., A.R. Nelson, S.M. Phillips, J.A. Faulkner, J. Clarke, N.A. Burd, D. Moore, and T. Stellingwerff (2015). Protein-leucine fed dose effects on muscle protein synthesis after endurance exercise. Med. Sci. Sports Exerc. 47:547-555.
Tipton, K.D., A.A. Ferrando, S.M. Phillips, D. Doyle Jr., and R.R. Wolfe (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 276:E628-E634.
Tipton, K.D., T.A. Elliott, A.A. Ferrando, A.A. Aarsland, and R.R. Wolfe (2009). Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl. Physiol. Nutr. Metab. 34:151-161.
Wilkinson, D.J., T. Hossain, D.S. Hill, B.E. Phillips, H. Crossland, J. Williams, P. Loughna, T. A. Churchward-Venne, L. Breen, S.M. Phillips, T. Etheridge, J.A. Rathmacher, K. Smith, N.J. Szewczyk, and P.J. Atherton (2013). Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 591:2911-2923.
Witard, O.C., S.R. Jackman, L. Breen, K. Smith, A. Selby, and K.D. Tipton (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 99:86-95.