Key Points
- Strength and muscle mass gains with training can be improved by optimizing nutrition.
- Both strength training and eating a meal rich in amino acids result in an increase in protein synthesis.
- The increase in protein synthesis in both cases is dependent on a protein kinase called the mammalian target of rapamycin (mTOR).
- Strength training and amino acid ingestion (eating) activate mTOR in different ways. As a result, when both are done together, the effects add up and result in a larger effect than either strength training or eating alone.
- Proteins that result in a rapid and prolonged (~1 hour) increase in the amino acid leucine in the blood maximize the activation of mTOR and the increase in muscle protein synthesis and strength.
- A simple nutritional strategy is presented that can be used to maximize the adaptive response to strength training.
Introduction
For thousands of years, we have understood the basic principle needed to overload muscle in order to increase strength. The earliest example of this principle was Milo of Croton. Milo was a farmer and Olympic wrestler. At the start of each Olympic training cycle he would select a calf from his herd and perform his morning exercises with the calf draped across his shoulders. As the calf grew so did Milo’s strength. When the Olympics arrived, he is said to have carried the then fully-grown bull on his shoulders into the stadium, thrown it to the ground and ate it. This fable is an illustration that what we know today has changed very little in 2,500 years: In order to increase strength we need to perform resistance exercise and then consume high-quality protein.
Over the last 2,000 years, a great deal of progress has been made in understanding how strength training and nutrition work together to increase muscle mass and strength. This Sports Science Exchange article will review that information and illustrate how to use it to improve the adaptation to training.
Molecular Response to Training
In every scientific model of muscle hypertrophy, from mice to rats, rabbits, chickens and, finally, to humans, the first response to a strength training session is an increase in protein synthesis. If the increase in protein synthesis is more than the increase in muscle breakdown, the muscle will get larger and stronger over time.
Over the past decade molecular exercise physiologists have shown that the single most important regulator of this training-induced increase in muscle protein synthesis is the mammalian target of rapamycin, or mTOR. If humans are given the immunosuppressant rapamycin (blocks mTOR) before they perform their strength training, there is no increase in protein synthesis (Drummond et al., 2009). This demonstrates that mTOR is required for the increase in muscle protein synthesis after resistance exercise. In support of the importance of mTOR, the phosphorylation of the protein S6K (a marker of mTOR activity) 30 min after strength training is the best predictor of the increase in muscle mass and strength that an athlete will achieve (Figure 1, Terzis et al., 2008). Even though there is more to muscle growth than activating mTOR, coaches and athletes should be trying to increase mTOR activity as much as possible.
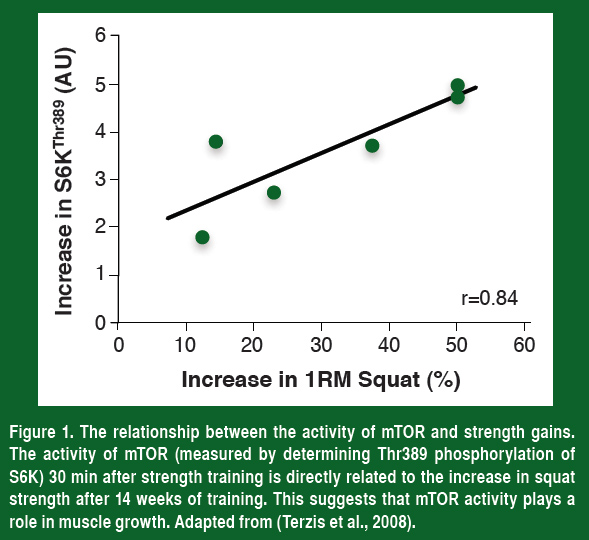
If activating mTOR is a key to increasing strength, then understanding how to maximally activate this enzyme is important to optimize our training. To do this, understanding what turns mTOR on and off is paramount, and from a number of excellent scientific studies, this is now becoming clearer.
At first, it was believed that the only way to increase mTOR activity was through growth factors like insulin growth factor 1 (IGF-1). Two results supported this: 1) insulin and IGF-1 both increased mTOR activity and this resulted in an increase in protein synthesis (Coleman et al., 1995), and 2) following resistance exercise there was an increase in the production of IGF-1 in the muscle (McKoy et al., 1999). From this data, it was believed that resistance exercise increased the production of IGF-1 and this led to the increase in protein synthesis. We now know that this was incorrect and that mTOR is activated directly by the mechanical load on the muscle and the IGF-1 plays a very small role in load-induced increase in muscle mass (Philp et al., 2011).
But the load on a muscle is not the only thing that leads to the activation of mTOR. While it is true that the heavier weight a person lifts, the greater the activation of mTOR (Baar & Esser, 1999; Terzis et al., 2010), it is now becoming clear that there is something more. When a lighter weight is lifted and the blood flow to the muscle is restricted (Fujita et al., 2007) or any weight is lifted to failure (Mitchell et al., 2012), mTOR activity and protein synthesis are turned on. Therefore, it is now believed that the activity of mTOR is maximally increased by strength exercises performed to failure. There are two types of failure in strength training. Positive failure, when an athlete can no longer lift a weight, and negative failure, when an athlete’s coach lifts the weight for them to lower and they can no longer slow the weight as it comes down.
Nutrition to Maximize Training
But increasing mTOR activity by lifting heavy weights is not enough. If an athlete trains in a fasted state, the increase in mTOR activity and protein synthesis is not optimal. In fact, in the fasted state, protein balance (protein synthesis minus protein degradation) remains negative after training (Figure 2). In order to maximally activate mTOR and protein synthesis and shift the muscle into a positive protein balance we need not only mechanical activation but the ingestion of amino acids as well.
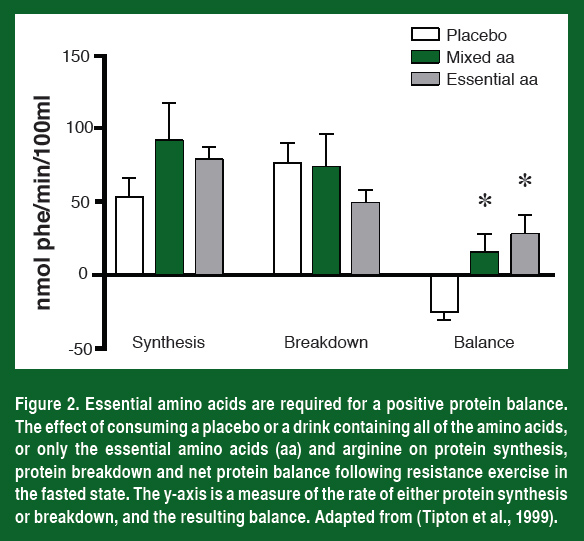
The amino acids serve two purposes in muscle. The first is to supply the building blocks necessary to synthesize new proteins and the second is to provide a signaling trigger that activates mTOR.
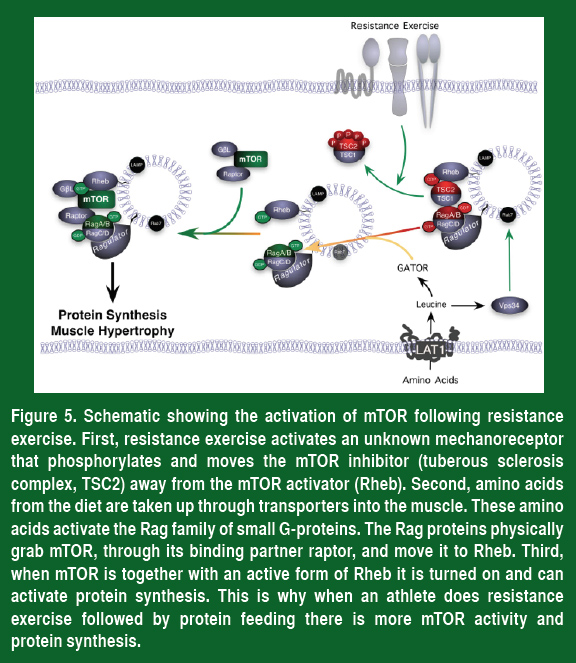
Even though all of the amino acids are needed to synthesize new proteins, only one is needed to turn on mTOR. This unique amino acid is the branched chain amino acid leucine. The reason that leucine is so important is that muscle cells contain a sensor to detect the levels of leucine. This amino acid sensor activates mTOR through a collection of transport proteins called the Rag proteins, which bring mTOR together with its activator Rheb. Therefore, when leucine enters muscle cells it turns on the Rag proteins and moves mTOR to Rheb, and this in turn activates mTOR, increases protein synthesis and makes larger, stronger muscles.
If leucine plays an important role in activating mTOR, then nutritionally it becomes important to understand how different protein sources affect the amount of leucine delivered to muscle. Work from Stuart Phillips’ laboratory (Tang et al., 2009) has shown that more leucine is released into the blood and that the leucine concentration stays higher for a longer time period when an athlete consumes whey compared to either soy or casein (Figure 3).
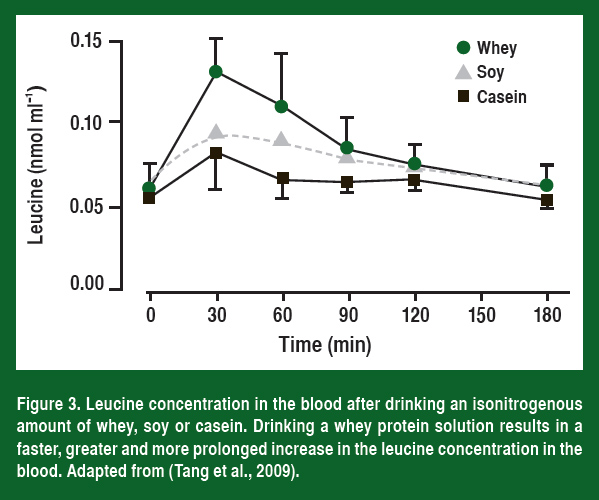
As would be expected from what has been discussed so far, the higher leucine levels resulted in a significantly greater increase in muscle protein synthesis both at rest and after resistance exercise (Figure 4). Most importantly, the researchers followed these individuals throughout training and have now shown that the athletes that drank a whey supplement right after they trained also had the greatest increase in muscle mass (Tang et al., 2009).
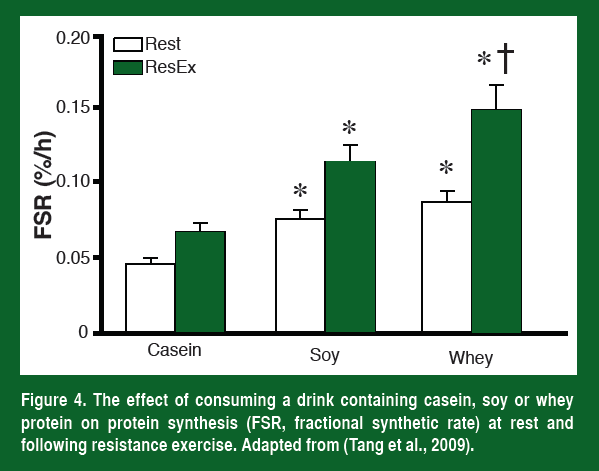
Leucine cannot get into muscle without a transporter. The primary leucine transporter in skeletal muscle is the L-type amino acid transporter LAT1 (also known as SLC7A5). In order to increase mTOR activity and protein synthesis LAT1 needs to increase in the plasma membrane. The current evidence suggests that immediately following strength training, LAT1 increases in the membrane for a short period of time (~90 min). However, LAT1 mRNA stays high for at least 24 h (Churchward-Venne et al., 2012; and Baar et al., unpublished). These facts could explain two things, 1) why taking amino acids immediately following resistance exercise is the most effective way to increase muscle protein synthesis (more LAT1 on the membrane to take up leucine, Esmarck et al., 2001), and 2) why eating amino acid-rich foods has a greater effect on muscle protein synthesis for 24 h after a heavy weights session (more mRNA that can be used to increase LAT1 and leucine uptake, Burd et al., 2011).
Science-based Recommendations for Training to optimize mTOR Activation and Strength Gains
Taking all of this information together, a simple plan can be devised to increase mTOR activity, protein synthesis and muscle strength (Figure 5).
-
To maximize strength gains, athletes should lift heavy weights to failure. This can be done using one set or more of each exercise. If using one set, the athlete needs to be lifting 100% of their 8RM until failure. Each additional set adds little to the final increase in strength since for each extra set the percent of 8RM decreases and failure is achieved only on the final set. This is why the strength gains achieved using one set are equal to that of multiple sets (Mitchell et al., 2012).
-
Immediately following resistance exercise, consume a rapidly absorbable protein source rich in the amino acid leucine. Examples of these types of food are dairy products (specifically the whey component) and eggs. Getting leucine into the blood quickly takes advantage of the higher LAT1 content at the membrane following resistance exercise.
-
In the 24 h after resistance exercise, eat meals containing 20 g of leucine-rich amino acids first thing in the morning and then every 3-4 h throughout the day. Twenty grams of amino acids maximally activates protein synthesis in young people (Moore et al., 2009).
-
Consume 30-40 g of leucine-rich protein right before bed. Eating right before bed improves protein synthesis while sleeping and maintains a positive protein balance overnight (Res et al., 2012).
The above program is likely to maximize strength gains. This article has focused on the role of mTOR in gaining strength, but this is not the only important factor. Muscle mass is also influenced by the growth inhibitor myostatin, the transcriptional regulator Notch and the number of satellite cells in the muscle. However, how these factors affect muscle size and strength and whether they are affected by nutrition is less certain.
References
Baar K & Esser K. (1999). Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276, C120-127.
Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK & Phillips SM. (2011). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of nutrition 141, 568-573.
Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K & Phillips SM. (2012). Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. The Journal of physiology.
Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C & Schwartz RJ. (1995). Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270, 12109-12116.
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E & Rasmussen BB. (2009). Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587, 1535-1546.
Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M & Kjaer M. (2001). Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 535, 301-311.
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E & Rasmussen BB. (2007). Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985)103, 903-910.
McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B & Goldspink G. (1999). Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516 ( Pt 2), 583-592.
Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK & Phillips SM. (2012). Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985) 113, 71-77.
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA & Phillips SM. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. The American journal of clinical nutrition 89, 161-168.
Philp A, Hamilton DL & Baar K. (2011). Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol (1985) 110, 561-568.
Res PT, Groen B, Pennings B, Beelen M, Wallis GA, Gijsen AP, Senden JM & van Loon LJ. (2012). Protein Ingestion Prior To Sleep Improves Post-Exercise Overnight Recovery. Medicine and science in sports and exercise.
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA & Phillips SM. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985)107, 987-992.
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H & Blomstrand E. (2008). Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102, 145-152.
Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P & Blomstrand E. (2010). The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. European journal of applied physiology 110, 835-843.
Tipton KD, Gurkin BE, Matin S & Wolfe RR. (1999). Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10, 89-95.