KEY POINTS
-
Vitamin D plays an important role in an athlete’s health, training and performance.
-
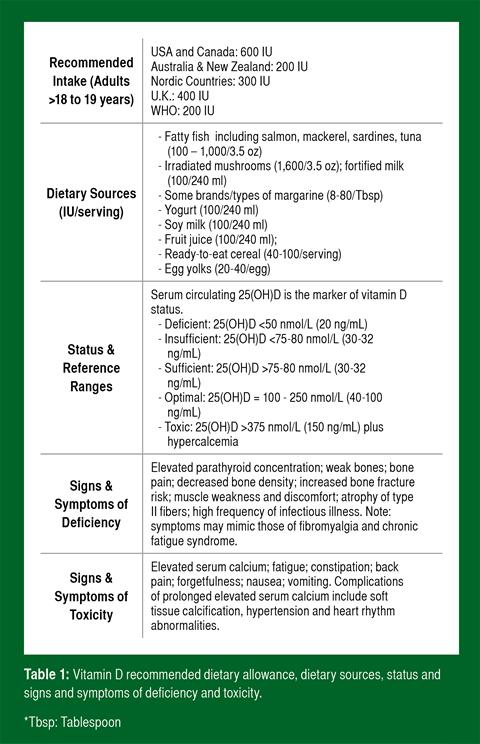
Vitamin D status should be assessed as part of a routine nutritional assessment. The blood 25(OH)D concentration is the best indicator of vitamin D status. A blood vitamin D concentration of >75 nmol/L but preferably >100 nmol/L should be maintained.
-
“Suboptimal” vitamin D status is linked to increased risk for acute illness, inflammatory injury, stress fracture, muscle pain/weakness and suboptimal muscle performance. Athletes with a history of these issues may benefit from assessment of vitamin D status.
-
Regular consumption of vitamin D-containing foods alone is not likely to maintain sufficient vitamin D status. Sensible sun exposure (5 to 30 min of exposure to arms, legs and back at close to solar noon several times a week), regular vitamin D supplementation (1,500-2,000 IU/day), or a combination of dietary intake, sun exposure and supplementation are required to achieve sufficient status.
-
In winter months, vitamin D supplementation is needed for athletes living at >35° north or south.
INTRODUCTION
It is long recognized that adequate vitamin D status is necessary for both bone and skeletal health. Emerging research, however, also indicates the important role of vitamin D for non-skeletal functions including skeletal muscle growth, immune function, inflammatory modulation and athletic performance (Larson-Meyer & Willis, 2010). In addition, research is finding that vitamin D deficiency increases risk for numerous chronic and inflammatory diseases including hypertension, cardiometabolic disease, arthritis and certain cancers (Holick, 2007) which can occur even in athletes. This Sports Science Exchange reviews vitamin D in the health and performance of athletes and provides tips for assessing and treating vitamin D insufficiency.
VITAMIN D SYNTHESIS, SOURCES AND INDICES OF STATUS
Vitamin D Synthesis and Metabolism
Although vitamin D is considered a “vitamin”—i.e., an organic compound in food needed in small amounts for growth and good health—the human requirement can be met entirely through synthesis in the skin upon exposure to sunlight (Holick, 2007). The ultraviolet-B (UVB) radiation in sunlight converts its precursor 7-dehydrocholesterol––present in the skin—to D3 (cholecalciferol). Newly synthesized vitamin D (as well as vitamin D obtained from the diet) is escorted to the liver by its carrier, vitamin D binding protein (VDBP). In the liver, vitamin D is rapidly converted to 25(OH)D, the main storage form. Further activation in the kidney tubules to 1,25(OH)2D, the hormonally active form, is driven by parathyroid hormone (PTH) when blood calcium and/or phosphate concentrations fall below the normal range.
Vitamin D Sources
Vitamin D is found in the diet from limited natural and fortified foods (Table 1). Dietary vitamin D includes both D3 (cholecalciferol) found naturally in specific animal foods and vitamin D2 (ergocalciferol) derived from UVB-exposed fungi and yeast ergosterols. Both forms are well absorbed (50% bioavailable) in association with dietary lipid. As such, vitamin D absorption is enhanced by high-fat meals (Raimundo et al., 2011) and may be limited by an extremely low-fat diet or by malabsorptive syndromes and conditions (Ross et al., 2010).
Indices of Vitamin D Status
Serum 25(OH)D concentration (hereafter referred to as blood vitamin D) is the best indicator of vitamin D status. The circulating concentration of the hormonally active form, 1,25(OH)2D, is dependent on factors other than status, including PTH and blood calcium and phosphate concentrations. Definitive cut-offs for vitamin D status are not yet scientifically established (as are other clinical thresholds such as serum calcium), but are based on clinical and disease risk markers. Table 1 summarizes the blood thresholds defined by most vitamin D researchers as “deficient,” “insufficient,” “sufficient,” “optimal” and “toxic” (Cannell et al., 2008; Holick, 2007; Hollis, 2005; Hossein-nezhad & Holick, 2013). The cut-off for deficiency is the approximate concentration at which PTH increases abruptly, while the cut-off for insufficiency is the concentration where PTH plateaus and calcium absorption is maximized. Lastly, the cut-off for optimal is the point where the human genome is believed to evolve. The Recommended Daily Allowance (RDA) for the United States and Canada is different than values proposed by researchers and was established using 50 nmol/L as an “adequate” level. In contrast, available evidence suggests that maintaining blood concentrations in the optimal range may be beneficial for skeletal muscle function (Bischoff-Ferrari, et al., 2004b) and disease risk (Cannell et al., 2008).
VITAMIN D INTAKE AND STATUS OF ATHLETES
Vitamin D Status of Athletes
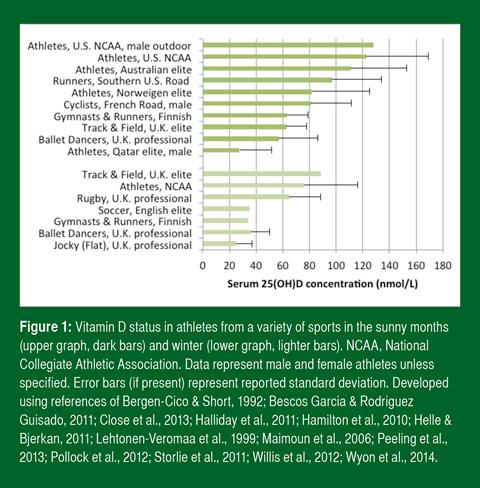
Suboptimal vitamin D status is widespread among the general population worldwide (Holick, 2007; Hossein-nezhad & Holick, 2013). In athletes, the prevalence of deficiency and sufficiency varies by season, training location, sport (Larson-Meyer & Willis, 2010) and skin color (Hamilton et al., 2010; Pollock et al., 2012; Shindle et al., 2011) (Figure 1). Vitamin D status is generally lower in the winter months (Farrokhyar et al., 2015; Halliday et al., 2011). Athletes who train predominantly indoors and who train at higher latitudes generally have lower status than those who train outdoors and at lower latitudes (Figure 1). Suboptimal vitamin D status, however, occurs even in sunny countries near the equator when sun is avoided or skin is shielded (Hamilton et al., 2010).
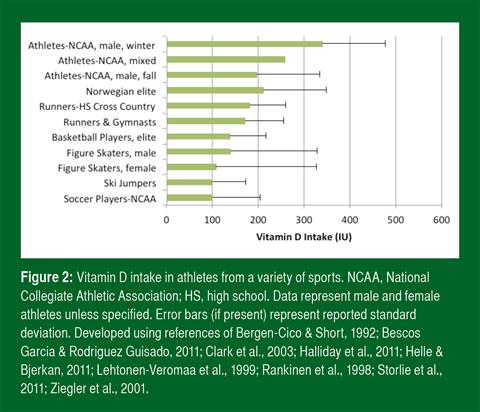
Vitamin D Intake in Athletes
Insufficient sunlight (UVB) exposure is the most probable reason for suboptimal vitamin D status. Poor vitamin D intake, however, may contribute. Studies find that athletes do not come close to meeting the dietary recommendations of most countries (Figure 2). One study found that only 5% of college athletes met the U.S. RDA from food alone (Halliday et al., 2011).
Factors Influencing Status
Because adequate sunlight is necessary for vitamin D synthesis, anything that limits the amount or quality of sun exposure can compromise vitamin D status (Table 2). In addition, body size and/or excess adiposity may compromise vitamin D status (Halliday et al., 2011; Heller et al., 2014). In athletes, it is not established whether this association is due to sequestration of vitamin D in excess adiposity or volume dilution in the larger body of bigger and/or overweight athletes. It is also not yet understood whether adipose-stored vitamin D is released during winter or periods of reduced UVB exposure.

FUNCTIONS OF VITAMIN D
As a secosteroid, (i.e., steroids in which one of the steroid rings is opened), vitamin D functions as a modulator of as many as 2,000 genes involved in cellular growth, immune function and protein synthesis (Cannell et al., 2009; Holick et al., 2011; Hossein-nezhad & Holick, 2013). In this role the active form of vitamin D (1,25(OH)2D) forms a complex with its nuclear vitamin D receptor and the retinoic acid α-receptor, and is able to “turn on” and “turn off” expression of specific genes (Holick, 2007). Current understanding is that an adequate concentration of vitamin D in blood is necessary to optimize genomic function (Hossein-nezhad & Holick, 2013). This role as a genetic modulator switch explains how vitamin D can play a role in a variety of physiologic functions, including bone health, muscle function, inflammation and immunity, all of which are important to health, training and performance.
Bone Health
Vitamin D influences bone health by turning on expression of genes that enhance intestinal calcium absorption, renal calcium resorption (in association with elevated PTH) and bone turnover (Holick, 2007). An example of the influence of vitamin D is found with calcium absorption which is >30% bioavailable when blood vitamin D concentration is at least 75 nmol/L but only 10-15% when concentrations are lower. Much of this effect is due to vitamin D-enhanced expression of intestinal proteins that promote calcium absorption. Moreover, research suggests blood vitamin D concentration is associated with bone mineral density (BMD) and/or bone mineral content in the hip and lumbar spines of women across their lifespan (Bischoff-Ferrari et al., 2004a).
Several studies provide evidence that sufficient vitamin D status is important for bone health and prevention of bone injury in athletic populations (Lappe et al., 2008; Ruohola et al., 2006; Valimaki et al., 2004). For instance, stress fracture risk was 3.6 times higher in Finnish military recruits with blood vitamin D concentrations below 75 nmol/L (Ruohola et al., 2006) and appeared to be protected by dietary vitamin D intake (> ~600-700 IU/d) in a mixed population of athletic and non-athletic U.S. adolescent girls (Sonneville et al., 2012). Supplementation with 800 IU of vitamin D daily (plus 2,000 mg/d calcium) for 8 weeks reduced stress fracture incidence by 20% in U.S. female Navy recruits (Lappe et al., 2008).
Skeletal Muscle Function
Musculoskeletal pain and weakness are well established but often forgotten symptoms of vitamin D deficiency that resolve with repletion. The latest research suggests that vitamin D influences skeletal muscle by turning on expression of genes that influence muscle growth and differentiation, particularly in fast-twitch (type II) fibers (Barker et al., 2011; Girgis et al., 2013). Vitamin D may also have non-genomic effects which include modulating sarcoplasmic calcium uptake and cell signaling. Animal and tissue culture studies show that vitamin D deficiency induces atrophy of fast-twitch muscle fibers, impairs sarcoplasmic calcium uptake and prolongs time to peak contractile tension and relaxation (Girgis et al., 2013). Biopsy studies in deficient patients show type II skeletal muscle fiber atrophy (Sato et al., 2005).
Low vitamin D status may directly impair muscle strength and performance in athletes, although evidence is limited. The first evidence that vitamin D influenced performance came from the turn of the 20th century when it was found that exposure to UVB light through a central sun lamp improved muscle performance (presumably by improving vitamin D status) (Cannell et al., 2009). Recent studies in younger (Ward et al., 2009) and older non-athletes find that low vitamin D status is negatively associated with markers of muscle strength up to a blood threshold of 94 nmol/L (38 ng/mL), when muscle strength plateaus (Bischoff-Ferrari, et al., 2004b). In deficient athletes, vitamin D supplementation at a dose which elevates blood vitamin D concentration also seems to improve select muscle performance parameters, including isometric quadriceps strength, vertical jump and 10-m sprint performances (which mostly recruit type II fibers) (Close et al., 2013; Wyon et al., 2014). In injured athletes and non-athletes, vitamin D insufficiency also seems to delay rehabilitation and recovery following orthopedic surgery (Barker et al., 2011; Kiebzak et al., 2007). Although not yet demonstrated in athletes, prolonged supplementation in deficient patients improves muscle strength and the relative size and number of type II muscle fibers (Sato et al., 2005).
Immunity and Inflammation
Immunity: Vitamin D functions as an important regulator of inflammation and innate immunity. In the innate immune system, vitamin D has the ability to turn on the gene expression of broad spectrum antimicrobial peptides (AMP) (Cannell et al., 2008; Larson- Meyer & Willis, 2010). AMPs are secreted by cells of the innate immune system, including monocytes, macrophages and epithelial cells in the respiratory tract (Gombart et al., 2005), and help defend against invading pathogenic bacteria, fungi and viruses. Because of the regulatory role of vitamin D on AMP expression in respiratory cells, it is suggested that vitamin D status (and its typical seasonal fluctuation) influences susceptibility to influenza and the common cold (Cannell et al., 2006). In athletes, it is well known that prolonged intense training has a suppressive effect on innate immune function and increases risk of upper respiratory tract infection. Vitamin D may also influence susceptibility to such infections in athletes (Larson- Meyer & Willis, 2010). A study in college athletes found that blood vitamin D levels during winter and springtime were negatively associated with upper respiratory illness during the season (Halliday et al., 2011). The breakpoint occurred at ~95 nmol/L such that athletes who maintained stores lower than the breakpoint had one or more episodes of illness whereas those with higher stores had one or fewer episodes. Another study in the Finnish military found that soldiers with blood vitamin D concentrations <40 nmol/L had 63% more absences from duty due to respiratory illness than those with higher status (Laaksi et al., 2007).
Inflammation: Vitamin D also works through the immune system to control inflammation, which is the accumulation of fluid and immune cells in injured tissue. Vitamin D increases the production of several anti-inflammatory cytokines including transforming growth factor and interleukins-4, -10 and -13 and reduces the production of pro-inflammatory cytokines such as interleukin-6, interferon-γ, interleukin-2 and tumor necrosis factor (TNF-α) (Barker et al., 2014; Larson-Meyer & Willis, 2010; Willis et al., 2012).
Currently, there is limited evidence which directly links compromised vitamin D status with increased risk or severity of sports-related inflammation or injury (Farrokhyar et al., 2015), or with overtraining syndrome which is thought to be driven by an interleukin-6 inflammatory response. This link, however, was first described in a 1950’s report which observed significant reduction in chronic pain due to sports injuries following a 6-week program of UVB therapy from a sun lamp (Cannell et al., 2009). Several recent studies have provided support (Barker et al., 2014; Shindle et al., 2011; Willis et al., 2012; Wyon et al., 2014). For example, in distance runners, vitamin D status was negatively associated with blood concentration of the pro-inflammatory marker TNF-α which was drastically elevated when vitamin D concentration dropped below ~80 nmol/L (32 ng/mL) (Willis et al., 2012). In American professional football players, vitamin D status was lower in players who became injured during the season compared to those without injury (50 vs. 63 nmol/L) (Shindle et al., 2011). Oral supplementation with 2,000 IU vitamin D/day reduced injury occurrence after 4 months in professional U.K. ballet dancers (Wyon et al., 2014).
Vitamin D Requirements for Athletes
The RDA for vitamin D in the U.S. and Canada is 600 IU for children and adults up to 70 years of age; and 800 IU for adults over 70 (Ross et al., 2010). Although the U.S. RDA is higher than the recommendations of other countries (Table 1), many vitamin D experts believe the U.S. RDA, which was established exclusively on bone health (Ross et al., 2010), is not sufficient for non-skeletal health benefits (Heaney & Holick, 2011; Holick et al., 2011) and optimal health and athletic performance (Cannell et al., 2009). In contrast to the RDA, the Endocrine Society recommends 1,500- 2,000 IU/day for individuals not getting adequate sun exposure to keep concentrations in the sufficient range (Holick et al., 2011). There is no evidence to suggest that the vitamin D requirements of athletes are different than the general population.
The recommendation to obtain 5 (in very fair-skinned) to 30 (in darker-skinned) min of sunlight exposure to arms, legs and back at close to solar noon several times a week without sunscreen (Cannell et al., 2008; Holick, 2007) usually leads to sufficient vitamin D synthesis and status. Athletes who do not achieve regular sun exposure need supplemental vitamin D or a combination of dietary intake and vitamin D supplementation. Consumption of vitamin D-fortified foods or an ordinary multivitamin alone is not likely to maintain sufficient status (>75 to 80 nmol/L).
Vitamin D Intoxication
An understanding about vitamin D toxicity is important because some athletes, coaches and trainers believe that “if a little is good, more is better.” Vitamin D intoxication from excess intake or supplementation, however, is extremely rare (Table 1). Typical reported cases involve unintentional consumption of extremely high doses, often from industry error (Cannell et al., 2008; Holick, 2007). On the other hand, doses of 10,000 IU/day for up to 5 months do not result in toxicity (Holick, 2007). Toxicity from sunlight or artificial UVB exposure is not possible because metabolic feedback loops direct production to inactive photoproducts with prolonged exposure (Ross et al., 2010).
CLINICAL ASSESSMENT, EVALUATION AND TREATMENT
Routine screening of vitamin D status may be useful in the athlete (Larson-Meyer & Willis, 2010). If routine screening is not possible, athletes with a history of stress fracture, frequent illness, bone and joint injury, skeletal pain or weakness, or signs of overtraining should be targeted. Careful attention should also be given to athletes with restrained eating patterns who spend the majority of time indoors (e.g., gymnasts, dancers, wrestlers) as they may be at increased risk for both vitamin D deficiency and poor nutrient intake. Although blood vitamin D concentration using a reliable assay is the most important biochemical parameter, blood PTH, alkaline phosphatase and other markers of bone health may provide additional information. Blood PTH concentration typically increases as blood vitamin D concentration falls below 25-50 nmol/L (Holick, 2007) and is independently related to bone density (Halliday et al., 2011) and stress fracture risk in athletes. Blood alkaline phosphate concentration is a marker of bone damage from vitamin D deficiency (osteomalacia) that is not seen with general low BMD or osteoporotic bone. Risk factors and symptoms of deficiency, including unexplained muscle pain and weakness, overtraining injury and frequent illness such as respiratory tract infections should also be considered along with medication use because some medications interfere with vitamin D absorption or metabolism (Cannell et al., 2008; Holick, 2007). Moreover, estimates of intake of vitamin D and other nutrients important to bone health and muscle function (i.e., magnesium and vitamins A, C and K) should be considered (Larson-Meyer & Willis, 2010).
Vitamin D recommendations can be individualized to each athlete’s blood vitamin D concentration, clinical symptoms, diet and belief system. Athletes with insufficient status require supplementation with at least 1,500-2,000 IU/day vitamin D to keep blood vitamin D concentration in the sufficient range (Holick et al., 2011) if sensible sun exposure is not possible or desired. Higher doses may be required in athletes with excess adiposity/body size or darker skin, or who take medications affecting vitamin D metabolism. Athletes with deficient status may benefit from short-term, high-dose “loading” regimens under the supervision of a physician (Holick et al., 2011).
PRACTICAL APPLICATIONS
-
Knowledge of blood vitamin D status in relation to season and the athletes’ training regimen may help athletes optimize performance and health.
-
Vitamin D status may be achieved by sensible sun exposure (5 to 30 min of exposure depending on skin pigmentation (5 min for fair skinned and 30 min for dark skinned) to arms, legs and back at close to solar noon several times a week) and/or supplementation and dietary intake to provide at least 1,500 – 2,000 IU/day.
-
Athletes who are unsure about status or who have a history of stress fracture, frequent illness, bone and joint injury, skeletal pain or weakness, or signs of overtraining should have their blood vitamin D status evaluated.
REFERENCES
Barker, T., T.B. Martins, H.R. Hill, C.R. Kjeldsberg, R.H. Trawick, L.K. Weaver, and M.G. Traber (2011). Low Vitamin D impairs strength recovery after anterior cruciate ligament surgery. JEBCAM 16:201-209.
Barker, T., T.B. Martins, H.R. Hill, C.R. Kjeldsberg, B.M. Dixon, E.D. Schneider, V.T. Henriksen, and L.K. Weaver (2014). Vitamin D sufficiency associates with an increase in anti-inflammatory cytokines after intense exercise in humans. Cytokine 65:134-137.
Bergen-Cico, D.K., and S.H. Short (1992). Dietary intakes, energy expenditures, and anthropometric characteristics of adolescent female cross-country runners. J. Am. Dietet. Assoc. 92:611-612.
Bescos Garcia, R., and F.A. Rodriguez Guisado (2011). Low levels of vitamin D in professional basketball players after wintertime: relationship with dietary intake of vitamin D and calcium. Nutr. Hosp. 26:945-951.
Bischoff-Ferrari, H.A., T. Dietrich, E.J. Orav, and B. Dawson-Hughes (2004a). Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am. J. Med. 116:634-639.
Bischoff-Ferrari, H.A., T. Dietrich, E.J. Orav, F.B. Hu, Y. Zhang, E.W. Karlson, and B. Dawson-Hughes. (2004b). Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr. 80:752-758.
Cannell, J.J., R. Vieth, J.C. Umhau, M.F. Holick, W.B. Grant, S. Madronich, C.F. Garland, and E. Giovannucci (2006). Epidemic influenza and vitamin D. Epidemiol. Infect. 134:1129-1140.
Cannell, J.J., B.W. Hollis, M. Zasloff, and R.P. Heaney (2008). Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 9:107-118.
Cannell, J.J., B.W. Hollis, M.B. Sorenson, T.N. Taft, and J.J. Anderson (2009). Athletic performance and vitamin D. Med. Sci. Sports Exerc. 41:1102-1110.
Clark, M., D.B. Reed, S.F. Crouse, and R.B. Armstrong (2003). Pre- and post-season dietary intake, body composition, and performance indices of NCAA division I female soccer players. Int. J. Sport. Nutr. Exerc. Metab. 13:303-319.
Close, G.L., J. Russell, J.N. Cobley, D.J. Owens, G. Wilson, W. Gregson, W.D. Fraser, and J.P. Morton (2013). Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J. Sports Sci. 31:344-353.
Farrokhyar, F., R. Tabasinejad, D. Dao, D. Peterson, O.R. Ayeni, R. Hadioonzadeh, and M. Bhandari (2015). Prevalence of Vitamin D inadequacy in athletes: A systematic-review and meta-analysis. Sports Med. 45:365-378.
Fu, L., F. Yun, M. Oczak, B.Y. Wong, R. Vieth, and D.E. Cole (2009). Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin. Biochem. 42:1174-1177.
Girgis, C.M., R.J. Clifton-Bligh, M.W. Hamrick, M.F. Holick, and J.E. Gunton (2013). The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocrin. Rev. 34:33-83.
Gombart, A.F., N. Borregaard, and H.P. Koeffler (2005). Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 19:1067-1077.
Halliday, T., N. Peterson, J. Thomas, K. Kleppinger, B. Hollis, and D. Larson-Meyer (2011). Vitamin D status relative to diet, lifestyle, injury and illness in college athletes. Med. Sci. Sports Exerc. 42:335-343.
Hamilton, B., J. Grantham, S. Racinais, and H. Chalabi (2010). Vitamin D deficiency is endemic in Middle Eastern sportsmen. Pub. Health Nutr. 13:1528-1534.
Heaney, R.P., and M.F. Holick (2011). Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 26:455-457.
Helle, C., and K. Bjerkan (2011). Vitamin D status blant norske toppidrettsutovere-OG Faktorer AV Betydning for vitamin D-status. Idrettsmedisinsk Hostkongress 38.
Heller, J.E., J.J. Thomas, B.W. Hollis, and D.E. Larson-Meyer (2014). Relation between vitamin D status and body composition in collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. E-pub ahead of print. PMID # 25028792.
Holick, M.F. (2007). Vitamin D deficiency. N. Engl. J. Med. 357:266-281.
Holick, M.F., N.C. Binkley, H.A. Bischoff-Ferrari, C.M. Gordon, D.A. Hanley, R.P. Heaney, M.H. Murad, and C.M. Weaver (2011). Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab.
Hollis, B.W. (2005). Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 135:317-322.
Hossein-nezhad, A., and M.F. Holick (2013). Vitamin D for health: a global perspective. Mayo Clin. Proc. 88:720-755.
Kiebzak, G.M., N.L. Moore, S. Margolis, B. Hollis, and C.G. Kevorkian (2007). Vitamin D status of patients admitted to a hospital rehabilitation unit: relationship to function and progress. Am. J. Phys. Med. Rehabil. 86:435-445.
Laaksi, I., J.P. Ruohola, P. Tuohimaa, A. Auvinen, R. Haataja, H. Pihlajamaki, and T. Ylikomi (2007). An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 86:714-717.
Lappe, J., D. Cullen, G. Haynatzki, R. Recker, R. Ahlf, and K. Thompson (2008). Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 23:741-749.
Larson-Meyer, D.E., and K.S. Willis (2010). Vitamin D and athletes. Curr. Sports Med. Rep. 9:220-226.
Lehtonen-Veromaa, M., T. Mottonen, K. Irjala, M. Karkkainen, C. Lamberg-Allardt, P. Hakola, and J. Viikari (1999). Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur. J. Clin. Nutr. 53:746-751.
Maimoun, L., J. Manetta, I. Couret, A.M. Dupuy, D. Mariano-Goulart, J.P. Micallef, E. Peruchon, and M. Rossi (2006). The intensity level of physical exercise and the bone metabolism response. Int. J. Sports Med. 27:105-111.
Peeling, P., S.K. Fulton, M. Binnie, and C. Goodman (2013). Training environment and Vitamin D status in athletes. Int. J. Sports Med. 34:248-252.
Pollock, N., P. Dijkstra, R. Chakraverty, and B. Hamilton (2012). Low 25(OH) vitamin D concentrations in international UK track and field athletes. S. Afr. J. Sports Med. 24:55-59.
Raimundo, F.V., G.A. Faulhaber, P.K. Menegatti, S. Marques Lda, and T.W. Furlanetto (2011). Effect of high- versus low-fat meal on serum 25-hydroxyvitamin D levels after a single oral dose of vitamin D: A single-blind, parallel, randomized trial. Int. J. Endocrinol. 809069.
Rankinen, T., S. Lyytikainen, E. Vanninen, I. Penttila, R. Rauramaa, and M. Uusitupa (1998). Nutritional status of the Finnish elite ski jumpers. Med. Sci. Sports Exerc. 30:1592-1597.
Ross, A.C., C.L. Taylor, A.L. Yaktine, and H.B. Del Valle (2010). Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press.
Ruohola, J.P., I. Laaksi, T. Ylikomi, R. Haataja, V.M. Mattila, T. Sahi, P. Tuohimaa, and H. Pihlajamaki (2006). Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J. Bone Miner. Res. 21:1483-1488.
Sato, Y., J. Iwamoto, T. Kanoko, and K. Satoh (2005). Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc. Dis. 20:187-192.
Shindle, M., J. Voos, L. Gulotta, L. Weiss, S. Roder, G. Kelly, S. Lyman, J. Lame, R. Barnes, and R. Warren (2011). Vitamin D status in a professional american football team. Med. Sci. Sports Exerc. 43:S340-S341.
Sonneville, K.R., C.M. Gordon, M.S. Kocher, L.M. Pierce, A. Ramappa, and A.E. Field (2012). Vitamin D, calcium, and dairy intakes and stress fractures among female adolescents. Arch. Pediatr. Adolesc. Med. 166:595-600.
Storlie, D.M., K. Pritchett, R. Pritchett, and L. Cashman (2011). 12-week vitamin D supplementation trial does not significantly influence seasonal 25(OH)D concentrations in male collegiate athletes. Int. J. Health Nutr. 2:8-13.
Välimäki, V.V., H. Alfthan, E. Lehmuskallio, E. Loyttyniemi, T. Sahi, U.H. Stenman, H.
Suominen, and M.J. Valimaki (2004). Vitamin D status as a determinant of peak bone mass in young Finnish men. J. Clin. Endocrinol. Metab. 89:76-80.
Ward, K.A., G. Das, J.L. Berry, S.A. Roberts, R. Rawer, J.E. Adams, and Z. Mughal (2009). Vitamin D status and muscle function in post-menarchal adolescent girls. J. Clin. Endocrinol. Metab. 94:559-563.
Willis, K.S., D.T. Smith, K.S. Broughton, and D.E. Larson-Meyer (2012). Vitamin D status and biomarkers of inflammation in runners. Open Access J. Sports Med. 3:35-42.
Wyon, M.A., Y. Koutedakis, R. Wolman, A.M. Nevill, and N. Allen (2014). The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: a controlled study. J. Sci. Med. Sport 17:8-12.
Ziegler, P., J.A. Nelson, A. Barratt-Fornell, L. Fiveash, and A. Drewnowski (2001). Energy and macronutrient intakes of elite figure skaters. J. Am. Dietet. Assoc. 101:319-325.